- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Uses and Applications
Uses and Applications
Magnetic separations are one of biotechnology’s most versatile separation methods since they can directly purify cells, viruses, proteins, and nucleic acids from crude samples. The rapid and gentle procedure, combined with its ease of scale-up and automation, offers distinct benefits over conventional separation processes. Magnetic particles suited for the envisioned goal and whose complex synthesis includes various fields of research are in the midst of this procedure.
The growing numbers of scholarly articles on magnetic materials and their many uses demonstrate the significant attention to magnetic materials as a universal tool. In terms of size, chemical composition, and surface chemistry, considerable progress has recently been made in manufacturing magnetic iron oxide particles. Furthermore, surface coatings of polymers, silica, biomolecules, and other molecules on magnetic particles can be changed to increase affinity to target molecules. The magnetic iron oxide particles have been widely used in diagnostic applications such as sample preparation and biosensing platforms, resulting in selectivity, sensitivity against target molecules, and ease of use in sensing systems.
Magnetic beads (particles), sometimes known as superparamagnetic beads, are made of particles of -Fe2O3 (maghemite) or Fe3O4 (magnetite) enclosed in various biocompatible polymers (polystyrene, dextrins, polyvinyl alcohol, silica, etc.). The beads are either spherical or amorphous, with diameters ranging from nano to micrometers. Superparamagnetic beads, unlike conventional ferromagnets, become magnetized only in a magnetic field and lose their magnetism when the field is removed. Because they do not attract each other outside of a magnetic field, they can be employed without fear of clumping.
Magnetic beads (particles) are an entirely different type of solid support matrices from beaded agarose or other porous resins. They display many unique advantages:
1.
Magnetic beads have a high binding capacity and yield
Magnetic beads, unlike other solid support matrices, do not have a porous center that can help in binding. However, their small size (compared to different matrices, magnetic beads have a diameter of 1 to 10µm) allows them to bind biomolecules more effectively and achieve maximum binding capacity. Magnetic beads do not require many biomolecules to generate consistent results. Due to the non-porous magnetic beads, the biomolecule will only bond to the exposed outer surface of the beads. As a result, magnetic beads do not necessitate many biomolecules to get consistent results. Magnetic beads also reduce sample loss during the purification washes and elution processes.
2.
Simple and quick
Compared to other isolation procedures, magnetic separation is a substantially faster and easier method of separating a biomolecule.
Magnetic resins are used to replace time-consuming, difficult, and expensive chromatographic techniques such as those based on agarose, cellulose, sepharose, and Sephadex.
In column-based procedures, the lysate is centrifuged or cleared, the supernatant is added to the column, the membrane or resin is washed with buffer through centrifugation or vacuum manifold, and the required biomolecules are eluted in an adequate volume of buffer. When using column-based technologies, processing multiple samples in academic research labs may require a significant quantity of hand pipetting. This pipetting can discourage differences in the target biomolecule yield between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields.
On the other hand, magnetic bioseparations do not require pre-clearing procedures because the risk of nonspecific binding of undesirable molecules is negligible. Since beads are frequently visible, they are easy to observe and trace as the purification process proceeds. They eliminate the requirement for columns or filters and time-consuming centrifugation and pipetting. They can also handle a large number of samples in a short time and rapidly adapt to the automated system.
3.
Cost-effective
Magnetic separation might be less expensive. Magnetic beads are more costly than agarose beads, but they are more cost-effective because the experiment requires a lot lower reagent volume, less labor, and fewer Lab consuming items. This method does not require expensive equipment or reagents, such as microcentrifuges or columns. Only a magnetic stand is needed. Sensitive
These beads can capture target biomolecules in samples with low concentrations of target biomolecules.
4.
Reproducible results
Magnetic beads produce exceptionally reproducible yields of pure protein due to minimum manipulation and fewer needed steps throughout the purification process.
BcMag™ Magnetic Beads are monodispersed and core−shell superparamagnetic beads. The beads are in uniform size, narrow distribution with a large surface area, and unique surface coating with excellent batch-to-batch performance. They are available in 1µm, 2.5 µm, and 5.0 µm sizes. The beads are manufactured using nanometer-scale superparamagnetic iron oxide as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide. The excellent hydrophilic properties of the beads make them less nonspecific binding. Unlike most commercially available magnetic beads based on hydrophobic materials such as polystyrene, silica provides an ideal chromatography separation medium for the purification of target molecules due to its mechanical and chemical stability.
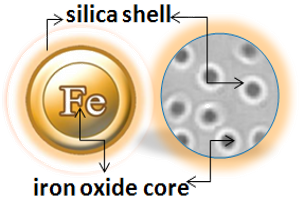
Bioclone has over 20 years of experience in magnetic separation technologies and offers a wide range of high-quality, consistent, and high-performance magnetic beads such as unique affinity magnetic beads (Preactivated magnetic beads and Pre-coupling affinity magnetic beads), Ion exchange magnetic beads, Hydrophobic chromatography magnetic beads, and Fluorescent magnetic beads. Because of their unique magnetic property, the beads have been widely used in a wide range of bioscience and medicinal applications such as high-throughput DNA/RNA, protein purification, proteomic screening, immunomagnetic isolations, and cell sorting. A wide range of sensitive and selective assays, ranging from the detection/separation/enrichment of antibodies, enzymes, proteins, and nucleic acids to entire cells, viruses, and pathogens, have been created, utilizing various signal transmission techniques such as fluorescence and so on.
Magnetic beads are an easy and dependable way to purify genomic, plasmid, and mitochondrial DNA. Classic silica-derived spin column and magnetic beads used for DNA and RNA purification are based on positive chromatography purifying selection. High chaotropic salt concentrations, such as guanidine hydrochloride, bind DNA or RNA to silica. The silica column or beads is washed with a salt/ethanol solution after nucleic acid binding to eliminate additional biomolecules from the sample. Finally, the column or beads is eluted using Tris elution buffer or water to remove the pure DNA or RNA. Such bind-wash-elute procedures are time-consuming, requiring multiple washing and spinning steps. Repetitive spin steps can cause considerable DNA loss (20-40%) and shearing. Furthermore, chaotropic salts and other impurities can easily pass through the eluted DNA or RNA, compromising ultimate purity and quantification as well as downstream enzymatic activities like PCR.
In contrast, negative chromatography purifying selection is a new nucleic acid purification technology that avoids the requirement for high salt binding and ethanol wash procedures, resulting in purer DNA and RNA preparations and more robust results. In negative chromatography, the multifunctional adsorbents efficiently capture and hold sample impurities such as protein, lipid, and ionic components in the sample while allowing nucleic acids to pass through the column, decreasing the number of steps and plastic materials required for purification. Additionally, since it does not touch the DNA and RNA molecules, no nucleic acids will get lost during the purification procedure.
Negative chromatography has three main advantages over positive chromatography:
●
Simplified workflow-Single step, fast
●
Superior performance- High quality and maximized DNA recovery with minimal loss of DNA
●
Waste reduction- Use fewer plastic tubes and no toxic substances.
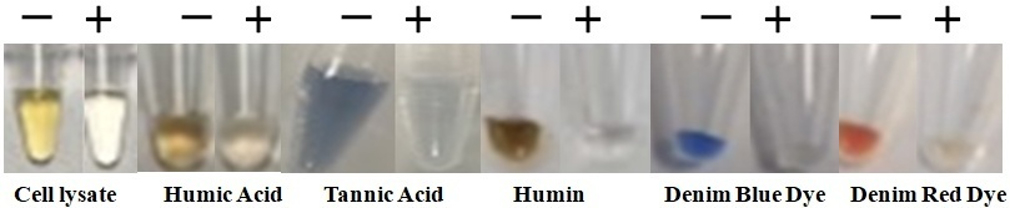
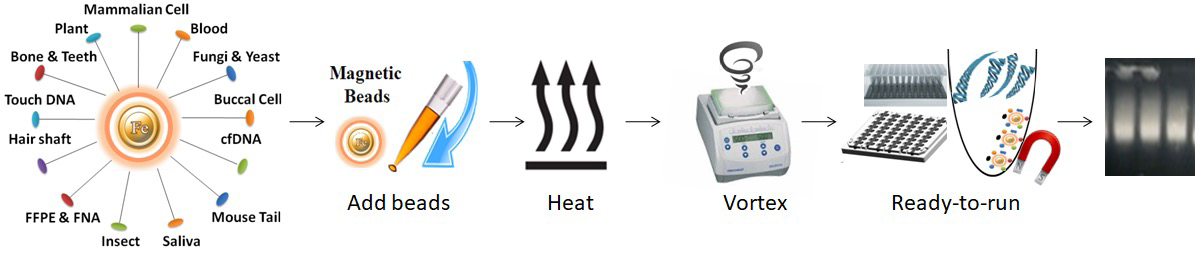
BcMag™ Onestep DNA Purification System uses novel Negative chromatography magnetic beads to quickly deliver higher quality and superior DNA yield from most DNA samples. Those samples include blood, animal cell, plants, body fluids, stains, swabs of body fluids, Strip removed cells, cigarette butts, Hair follicles, fingernail scrapings, epithelial cells, bite marks, semen, touch DNA samples, etc. The specially designed magnetic beads with our proprietary surface chemistry function simultaneously to lyse cells and capture the PCR inhibitors once mixed with the sample. The magnetic beads-PCR inhibitor complex was then magnetically removed by a magnet while the pure DNA remaining in the solution was ready for downstream STR analysis.
Features and Advantages
●
Rapid and efficient purification protocol: without prior DNA isolation for subsequent use in direct amp workflows, No liquid transfer, and One-tube
●
Ultrafast: Process 96 samples in less than an hour
●
Higher purity and DNA yield with minimal contamination with RNA from various trace samples.
●
Effectively removes inhibitors: polyphenolic compounds, humic/fulvic acids, acidic polysaccharides, tannins, melanin, heparin, detergents, denim dyes, divalent cations such as Ca2+, Mg2+, etc.
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High throughput: Compatible with many different automated liquid handling systems.
Workflow
Learn more
Affinity chromatography is a potent technique for purifying a specific molecule or a group of compounds from complex biological materials. It is based on reversible and highly specific contacts between the target molecule and an affinity ligand of a solid matrix, such as interactions between antigen and antibody, enzyme and substrate, or protein and nucleic acid. Because of its high selectivity and capacity, affinity chromatography not only allows for a fast, single-step process with potential for purification in the order of several thousand-fold but also can concentrate substances present at low concentration in large volumes of crude samples and separate active biomolecules from denatured or functionally distinctive forms.
Preactivated magnetic beads
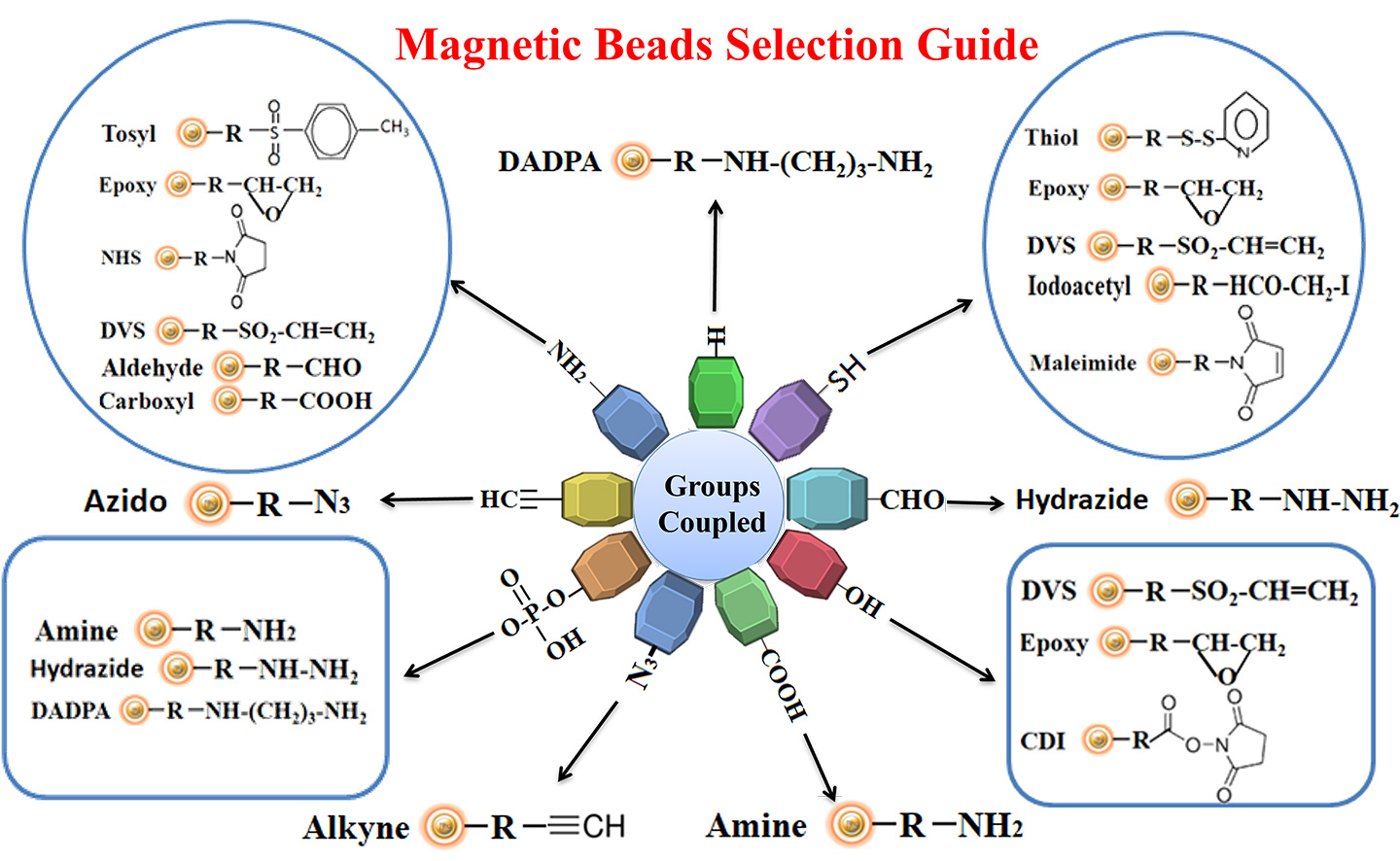
Making ligand-specific affinity supports requires methods to covalently conjugate compounds to an appropriate insoluble support (matrix). Immobilization or conjugation refers to the covalent attachment of a compound to a solid support through their common chemical groups. This method prepares the matrices by activating reactive chemical compounds toward one or more of these functional groups. These chemical groups should be easily activated, such as primary amines, sulfhydryls, aldehydes, carboxylic acids, etc. The activated matrices then can conjugate the compound through a covalent linkage, resulting in compound immobilization.
We provide a variety of active supports and accessories for protein, antibody, and other molecular immobilization. These resins can be purchased independently or as part of convenient kits.
Learn more
For convenience, base magnetic beads with ligands already coupled are supplied. Each ligand has a distinct affinity and can be utilized for different applications. Bioclone offers the following unique pre-coupled magnetic beads.
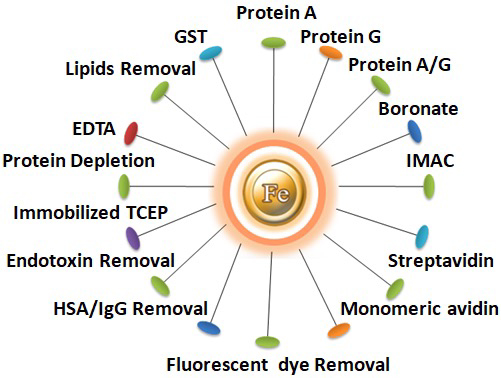
Learn more
Sample preparation is the most critical step in every analytical technique, such as food analysis, bioanalysis, forensics, toxicology, environmental monitoring, and so on. Many different contaminants are present in or created throughout the biological sample preparation. These contaminants include DNA, RNA, lipids, endotoxin, detergents, toxic metal ions, fluorescent dyes, and host cell proteins. These contaminants interfere with downstream applications like mass spectrometry (MS) and amino acid sequencing, antigen-antibody binding, immunoprecipitation assay, and ELISA. Before downstream applications, the impurities must be removed using a variety of chemical and physical purification processes. Examples are absorption, prolonged dialysis, chromatography, distillation, extraction, ion exchange, filtering, complex creation, crystallization, drying, and many other processes. However, these procedures are either laborious or suffer from sample losses and are challenging for low-volume samples and high thorough-put automation.
One-Step Sample Preparation Magnetic Beads
One-Step Sample Preparation Magnetic Beads (Fig.6) are activated macroporous beads that are used to efficiently remove >90% of target biomolecules (impurities) from biological samples. The protocol is straightforward and uses a one-step, one-tube protocol, leading to product recovery greater than 90%. The beads are customized with diverse chemistries to trap various target biomolecules (impurities). The robust beads offer different complementary solutions for efficiently removing impurities from DNA/RNA and protein/peptide samples. Due to magnetic characters, the beads can significantly reduce the time and effort expended upon purification and facilitate parallel application and automation.
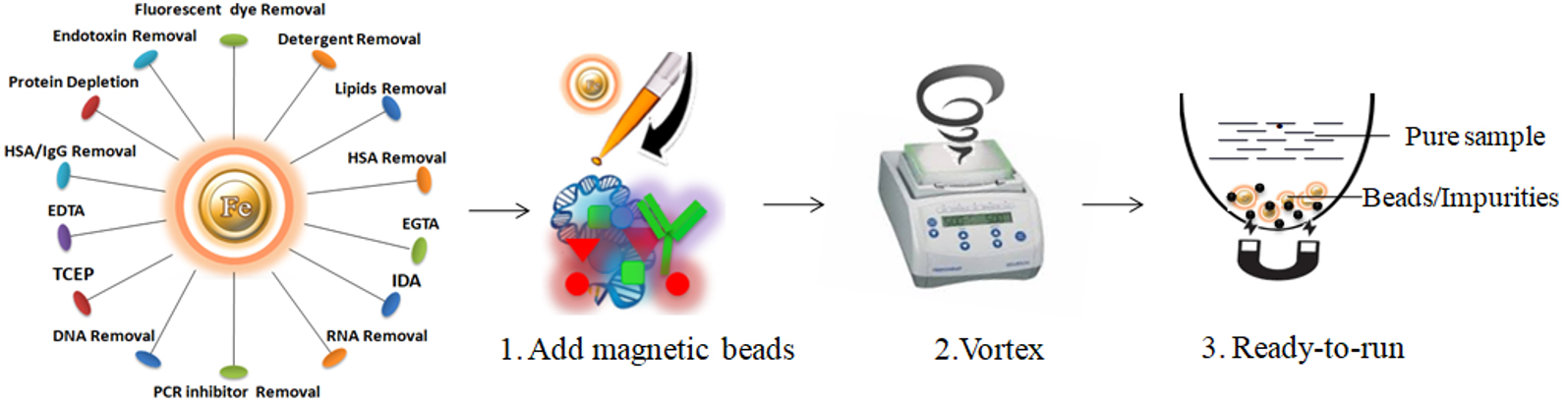
Learn more
Ion exchange chromatography (IEX) separates ionizable compounds according to their total charge. Because the charge on the molecule of interest is easily modified by adjusting buffer pH, this technique allows for separating similar types of molecules that would be hard to separate using other methods. Ion exchange chromatography is a powerful separation technique for preparative and analytical chromatography. It is widely used to separate and purify many charged or ionizable molecules such as proteins, peptides, enzymes, nucleotides, DNA, antibiotics, vitamins, etc.
Ion exchange chromatography is a compelling separation technology utilized not only for preparative chromatography but also for analytical chromatography. However, IEX, like all other chromatographic modes, has some restrictions.
One of the main limitations of ion-exchange chromatography is its buffer requirement. Because binding to IEX resins is based on electrostatic interactions between proteins of interest and the stationary phase, IEX columns must be loaded in low-salt buffers. This restriction may necessitate a buffer exchange step before ion-exchange chromatography in some applications.
We offer strong cation exchange (SCX), strong anion exchange (SAX), weak cation exchange (WCX), and weak anion exchange (WAX) magnetic beads (Table 1). Our ion exchange (IEX) beads are rigid polymeric beads with covalent surface chemistries, which allow for easier handling and packing while providing greater physical and chemical stability—resulting in a robust production process. The beads enable instant and efficient capture of the target molecules. The physical and chemical properties of the beads will allow them for efficient high-throughput purification in parallel and make the best performance and highly reproducible results in both manual and automated instrument-based assay and separation systems.
Table 1 – Ion Exchange Magnetic Beads & Features
Beads
Feature
●
A Strong Anion Exchange (SAX) Beads, pKa =~12
●
Used to extract weaker cations that may not bind to WAX Magnetic Beads
●
A Weak Anion Exchange (WAX) Beads, pKa=~11.5
●
Used to extract very strong cations that may be retained irreversibly to SAX Magnetic Beads
●
A Weak Cation Exchange (WCX) Beads, pKa =~4.7
●
Used to extract very strong Anions that may be retained irreversibly to SCX Magnetic Beads
●
A Strong Cation Exchange (SCX) Beads, pKa =~2.3
●
Used to extract weaker Anions that may not bind to WCX Magnetic Beads
●
A Mixed-mode Ion Exchange Magnetic Beads
●
Hydroxyapatite has two adsorbing sites; a calcium site that binds with the acidic groups and a phosphate site that interacts with basic protein groups.
Learn more
Hydrophobic interaction chromatography (HIC)
Hydrophobic interaction chromatography (HIC) is a technique for separating macromolecules from one another based on a reversible interaction between the external hydrophobic region of a biological macromolecule and the hydrophobic ligand (such as phenyl, octyl, or butyl) of a HIC medium (Fig.7). The interaction is enhanced by a buffer with a high salt concentration and reduced with a low salt concentration. Therefore, based on salt concentration in a buffer, the protein with less hydrophobicity is eluted first, whereas the protein with more hydrophobicity elutes last. Compared with other chromatography methods, HIC is a more popular method for separating and purifying protein and peptides in analytical and preparatory scale applications since it employs a less denaturing environment.
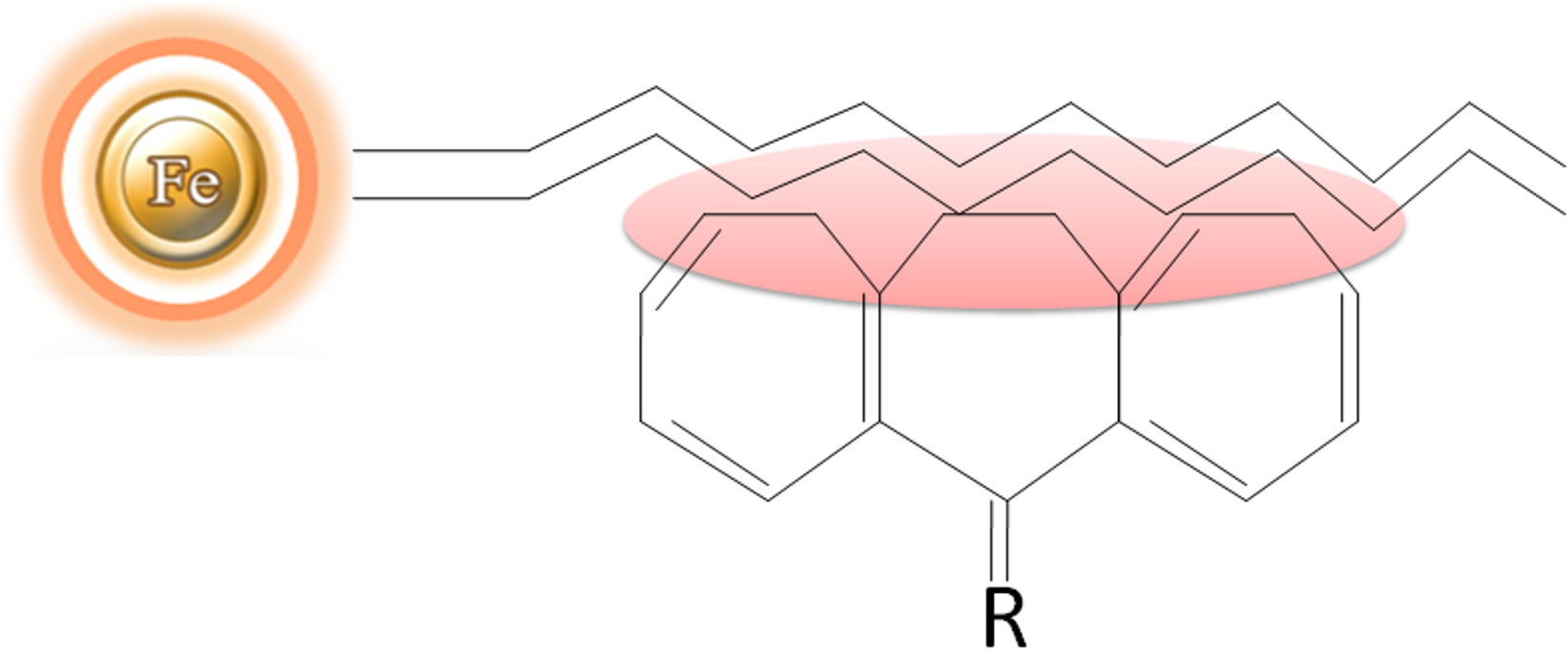
Reverse-phase chromatography (RPC)
Reverse-phase chromatography (RPC) is also used to separate proteins and peptides based on hydrophobic interaction chromatography. However, the ligands of an RPC medium are more hydrophobic than that of a HIC. This interaction is usually so strong that only organic solvents (e.g., acetonitrile or methanol) can successfully elute the proteins/peptides. Since organic solvents can denature many proteins, RPC is not recommended for protein purification.
BcMag™ hydrophobic magnetic beads
All BcMag™ hydrophobic magnetic beads are designed as uniform magnetic beads grafted with a high density of hydrophobic ligands on the surface. Our hydrophobic magnetic resins are rigid polymeric beads with covalent surface chemistries, allowing easier handling and packing while providing more excellent physical and chemical stability—resulting in a robust production process. The magnetic resins replace time-consuming, difficult, and expensive chromatographic techniques such as agarose, cellulose, sepharose, and Sephadex-based columns or resins. The hydrophobic magnetic beads are manufactured using nanometer-scale superparamagnetic iron oxide as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide. Pure inert silica makes less nonspecific binding. The beads are much smaller (1, 2.5, and 5 µm diameter) in size and are non-porous, which exhibit larger surface area, less nonspecific binding, and higher resolution than porous supports
These beads can be used in aqueous solutions and solvents such as alcohols, petroleum ether, diethyl ether, and hexane, as well as solvent mixes, without the resins expanding or contracting. Figure below is a Typical MALDI-TOF MS spectra (1-10 kDa) of LMW serum fraction processed on 1um C18 magnetic beads. (Dr. Radoslav (Rado) Goldman, Georgetown University Medical Center ).
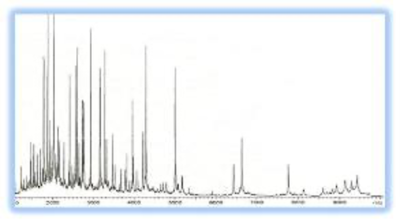
Table 2 – Explore The Products
Beads
Feature
●
Suitable for fractionation of larger molecular weight proteins and peptides. Use this less retentive phase to release hydrophobic molecules by weaker organic solvents rapidly.
●
Good matrix for fractionation of very large hydrophobic molecules which are bound too strongly to C-18 beads to be eluted.
●
The least selective phase binds most organic analytes from aqueous matrices. Beneficial for extracting numerous analytes diverse in structure for the same sample.
●
Ideal for very hydrophobic analytes that may be irreversibly retained on more hydrophobic C-18 beads.
●
Similar in polarity to C8 beads. However, an electron-dense aromatic ring offers some unique selectivity and retention.
●
Used for polypeptides with aromatic side chains, large, hydrophobic proteins, membrane-spanning
●
Peptides, lipid peptides, fusion proteins from inclusion bodies
Learn more
Fluorescent probes, also known as fluorescent labels, fluorescent tags, and fluorophores, are compounds that absorb light at high energy, short-wavelength (Ex: the Excitation), and emit light at lower energy, a longer wavelength (Em: the emission). This produces fluorescence in various colors that can be detected and analyzed by Fluorescent microscopes, Fluorescence scanners, such as microarray readers, spectrofluorometers and flow cytometers. Due to their high sensitivity, selectivity, safety, and fast response in target detection, fluorescent probes are widely used in life science such as immunochemistry, histochemistry, fluorescence in situ hybridization (FISH), cell tracing, single-dye oligos receptor for fluorescence-based sequencing, genotyping primers, probes for oligonucleotide ligation assay, SNP detection on microarrays, and other biological applications.
Fluorescence measurement can be divided into two categories: steady-state fluorescence and time-resolved fluorescence. The main difference between them is the timing of the excitation/emission process. Unlike Steady-State fluorescence resulting from the use of regular fluorescent dyes (e.g. fluorescein, rhodamine, etc.) whose Excitation and emission occur almost simultaneously (measured in nanoseconds), time-resolved fluorescence (TRF) relies on the use of fluorescent rare-earth chelates such as europium ion (Eu3+) or terbium (Tb3+) (also called lanthanide chelate labels), which allow emitting long-lived fluorescence (measured in milliseconds) after Excitation.
BcMag™ Time-resolved Fluorescence Magnetic Beads
Bioclone offers three unique Time-resolved Fluorescence Magnetic Beads: BcMag™ Europium Fluorescent Magnetic Beads, BcMag™ Terbium Fluorescent Magnetic Beads, and BcMag™ Ruthenium Fluorescent Magnetic Beads (Fig.9). They are uniform and monodisperse magnetic beads available in nominal diameters of 2.5µm and 5µm. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and Europium (Eu3+ cryptate) or Terbium (Tb3+ cryptate), or Ruthenium (Ru2+ cryptate) metal as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide and the metal. Their fluorescence properties are summarized in table 3.
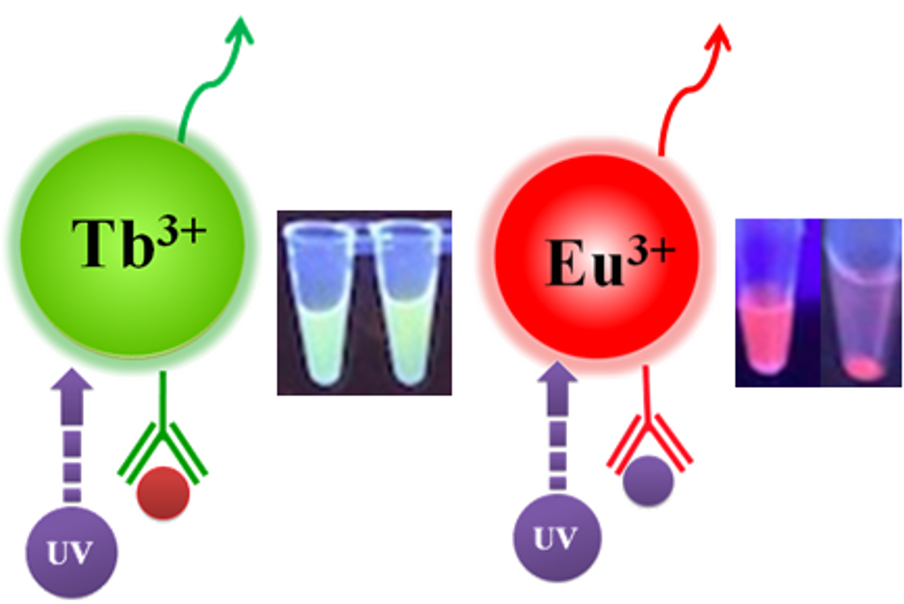
Table 3 – Fluorescent Properties Of TRF Magnetic Beads
Fluorophore
Fluorescent color
Stokes shifts
(nm)
Europium ion (Eu3+)
Red
340
615
730
275
Terbium (Tb3+)
Green
320
545
1050
220
Ruthenium (Ru2+)
Far-Red
470
710
354.36
175
Fluorophore
Europium ion (Eu3+)
Fluorescent color
Red
340
615
Fluorescence lifetime (Ԏ) (µsec)
730
275
Fluorophore
Terbium (Tb3+)
Fluorescent color
Green
320
545
Fluorescence lifetime (Ԏ) (µsec)
1050
220
Fluorophore
Ruthenium (Ru2+)
Fluorescent color
Far-Red
470
710
Fluorescence lifetime (Ԏ) (µsec)
354.36
175
Learn more
A magnetic separation rack (also called a separator or stand) separates these complexes of magnetic beads and their bound components from a complex mixture in solution. This procedure produces an isolated solution of your desired biological constituents, which can then be enhanced and concentrated.
The magnetic separator, which will separate your beads from the solution, is another critical factor in the magnetic bead separation procedure. An external magnetic field is necessary to move the beads, commonly referred to as a magnetic separation rack. Biomagnetic separation techniques are optimized by establishing the optimal magnetic bead and separation rack characteristics.
Magnetic force is a fundamental property of the biomagnetic separation process. Two significant forces are influencing your solution. The drag force caused by the viscosity of your solution stops your molecules from separating. The magnetic force of your magnetic separator rack overcomes the drag. Magnetic bead separation is most efficient when the separation conditions (magnetic force) are uniform across the working volume. As a result, it is vital to ensure that the separation rack can expand in tandem with the operational volume.
Bioclone offers different magnetic racks to meet different size of sample processing.
●
BcMag™ separator-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ separator-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ separator-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat.# MS-03)
●
BcMag™ separator-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
●
BcMa™ 96-well Plate Magnetic Separator (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible separators (Blioclone, Cat. No. MS-06)
Magnetic Beads Make Things Simple