- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

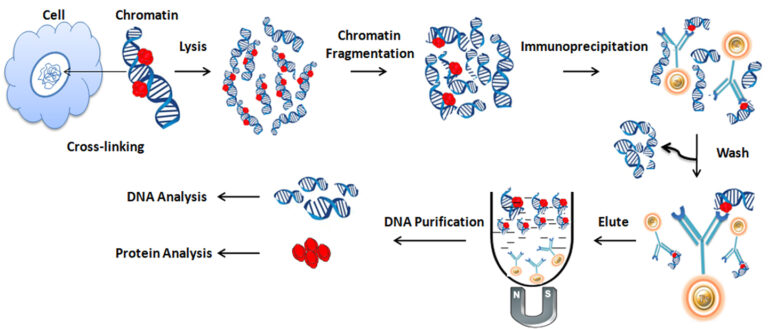
Chromatin Immunoprecipitation (ChIP) is an antibody-based affinity purification of the specific DNA-binding proteins and their targets (the protein–DNA complex) from the cellular lysates. The isolated DNA fragments can be further analyzed by PCR or qPCR, microarray, and Next Generation Sequencing (NGS) technologies. ChIP is a powerful technique to investigate the interaction between DNA and particular transcription factors or histone proteins and to study chromatin structure and nuclear events involved in transcription. In addition, it can identify the relative abundance of a specific protein and the particular genomic binding site(s) of various histone modifications (including acetylation, phosphorylation, or methylation). This technique is now widely used in life science, including cell differentiation, epigenetic gene silencing, and the effect of histone modifications on gene expression.
1.
Crosslinking
Formaldehyde is usually used to crosslink protein and DNA at a particular site. Optimizing the crosslinking reaction time is necessary since excessive crosslinking may reduce antigen accessibility and sonication efficiency.
2.
Chromatin Fragmentation
For efficient immunoprecipitation, sonicating chromatin into small fragments (average size of 200-1000bp) is necessary. Again, optimizing the sonication condition is essential for individual applications to determine the resolution of genomic mapping.
3.
Immunoprecipitation
Selecting an appropriate antibody for ChIP assays is critical for its success. The best choice for ChIP assays should be a ChIP- or ChIP-seq-validated antibody. The antibody should have high specificity and a strong affinity for the protein of interest. In addition, it is also vital to carefully choose an appropriate antibody binding protein, such as protein A/G or protein L, that matches the selected antibody.
Bead Choice
●
Magnetic vs. agarose beads
Magnetic beads (particles) are an entirely different type of solid support matrices from beaded agarose or other porous resins. They are much smaller (typically 1-5 µm diameter), thus providing larger surface areas for a high density of ligand immobilization. They are easy separation and visibility in the tube, leading to less loss of material and highly reproducible results. In addition, the beads are compatible with the exited automation system. Agarose beads have a relatively higher capacity for binding due to their porous nature. However, they have higher nonspecific binding.
●
Protein A vs. protein G vs. protein A/G vs. protein L
Protein A/G has a broader binding range than Protein A or Protein G individually. While Protein L binds a broader range of antibody classes than Protein A or G since the heavy chain is not involved in the binding interaction. Protein L binds to most Ig classes, single-chain antibody fragments (scFv), and Fab fragments.
The antibody to the protein of interest or the antibody immobilized to the protein A/G magnetic beads is then mixed and incubated with the chromatin mixture at 4oC to form bead/antibody/protein/DNA complexes. It is necessary to wash the beads to remove the nonspecific binding by magnet rack. The antibody/protein/DNA complex is finally eluted with SDS and heat from the beads and collected for further DNA purification.
Protocols for Immunoprecipitation
4.
DNA Purification
Before DNA purification, it is necessary to use heat, NaCl, and proteinase K to reverse the crosslinks and digest all the associated proteins and antibodies. A commercial DNA purification kit can purify the DNA.
5.
Analyze the Enriched DNA
The purified DNA is then analyzed by ChIP-PCR, ChIP-qPCR, ChIP-chip, and ChIP-seq to identify and quantify the sequences.
One-Step vs. Multistep PCR Purification
Chromatin immunoprecipitation followed by sequencing analysis (ChIP-Seq) is a powerful method to align purified DNA with previously annotated whole genomes to investigate genome-wide distributions of chromatin-binding proteins and histone modifications. Library construction for next-generation sequence (NGS) typically involves a multistep process including end-repair, single A-addition, adapter-ligation, size selection, gel purification, and PCR using primers specific to the sequencing platform. PCR purification is time-consuming in constructing a library, especially for handling multiple samples.
Unlike the traditional “Bind-Wash-Elute” multistep PCR purification method, BcMag™ Onestep PCR Cleanup Magnetic Beads use only one step, one minute, and one tube to finish the purification. The beads enable 96 samples to be processed simultaneously in less than 10 minutes. The beads can remove the impurities (e.g., excess primer, dimer, adapter, salt, detergent, dNTPs, and enzyme) and remove the different size DNA fragments by adjusting processing time, buffer pH, and detergent concentration.
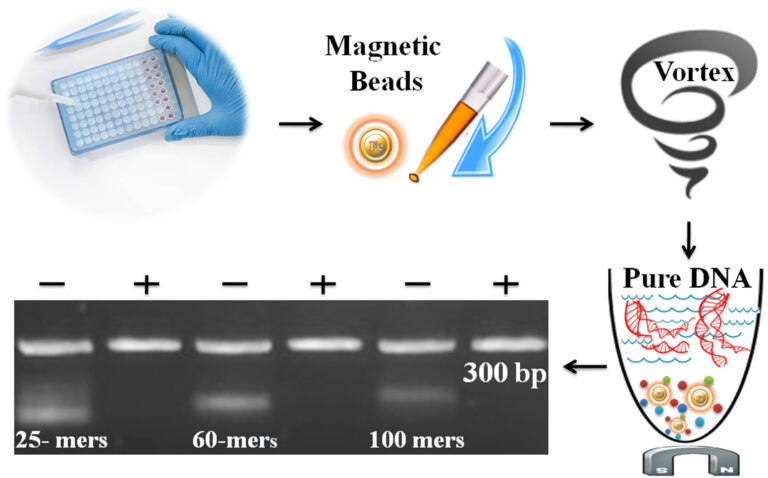
Magnetic Beads Make Things Simple