- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
MAL101
BcMag™ Protein L Magnetic Beads Purification Kit
Kit components:
– 2.0 ml BcMag™ Protein L magnetic beads
– 25 ml 5x Binding/Washing Buffer
– 2.0 ml 1x Elution Buffer
– 2.0 ml 1x Neutralization
Each
Specification
Beads Size
~ 2.5 μm diameter
Number of Beads
Magnetization
~ 40 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.5 g/ml
Stability
Short term: pH 3-11; Long term: pH 4-10
Binding Capacity
Storage
Store at 4ºC upon receipt
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit components according to the table above on arrival.
The BcMag™ Magnetic Beads provide a fast, efficient, and convenient chromatography method for separating biomolecules such as DNA, RNA, proteins, lipids, and carbohydrates from the complex biological sample in both manual and automated workflows.
BcMag™ Protein L Magnetic Beads are magnetic microspheres covalently immobilized with a high density ultrapure (Purity>95%) recombinant protein L. The beads are typically used for antibody isolation from serum, cell culture supernatant, ascites, immunoprecipitation, and co-immunoprecipitation of antigens from cell or tissue extracts. For antibody purification, the beads are incubated with the antibody solution, which is magnetically separated from the supernatant. For immunoprecipitation, the beads catch the antigen-antibody complex from the sample. The attached antibodies and antigens are dissociated from the beads using an elution buffer and recovered from the solution manually using a magnetic stand or by using automation instruments.
Protein L is a 36 kDa immunoglobulin-binding protein isolated from the anaerobic species Peptostreptococcus magnus but is now produced recombinantly in large amounts by E.coli. The recombinant Protein L contains only the IgG-binding domain; the other binding domains (Albumin, cell wall, and cell membrane) have been removed to ensure the maximum and specific binding properties. Protein L binds the different antibody isotypes (IgG, IgM, IgA, IgE, and IgD) through the interaction with the variable domain of the Ig kappa light chain with no interference with an antibody’s antigen-binding site. Compared with Protein A, Protein G, or Protein A/G, Protein L binds a broader range of antibody classes than Protein A or G since the heavy chain is not involved in the binding interaction. Protein L binds to most classes of Ig and single-chain antibody fragments (scFv) and Fab fragments. Table1 lists Protein L binding properties. Therefore, Protein L is mainly used to purify monoclonal antibodies from ascites or cell culture supernatant containing the kappa light chain. Protein L is a unique tool for purifying VLκ-containing monoclonal antibodies from culture supernatant since it does not bind bovine immunoglobilins, which are often present in the media.
Table 1. Protein L Antibody binding properties
Species
Antibody
Binding
(Protein L)
Mouse
IgG 1
IgG 3
IgG 2a
IgG 2b
IgM
Total IgG
++++
++++
++++
++++
++++
Human
IgG1
IgG2
IgG3
IgG4
IgA
IgD
IgM
Fab
scFv
Total IgG
++++
++++
++++
++++
++++
++++
++++
++++
++++
++++
Rat
IgG 1
IgG 2a
IgG 2b
IgG 2c
Total IgG
++++
++++
++++
++++
++++
Species
Antibody
Sheep
IgG1
IgG2
Total IgG
–
–
–
Horse
IgG(ab)
IgG(c)
IgG(T)
Total IgG
N/A
N/A
N/A
N/A
Goat
IgG1
IgG2
Total IgG
–
–
Cow
IgG1
IgG2
Total IgG
–
–
–
Rabbit
Total IgG
++
Guinea Pig
Total IgG
N/A
Pig
Total IgG
++++
Cat
Total IgG
N/A
Dog
Total IgG
N/A
++++ (Strong Binding)
+++ (Medium Binding)
++ (Weak Binding)
– (No Binding)
N/A (No Information)
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Binds IgG, IgM, IgA, IgE and IgD
●
Binds single-chain variable fragments (ScFv)
●
Not bind bovine, goat, or sheep Igs
●
Ultra-low nonspecific binding thanks to the beads’ hydrophilic surface
●
High binding capacity Scalable – easily adjusts for sample size and automation – low cost
●
Immunoprecipitation including IP, Co-IP, ChiP, RIP
●
Cell sorting
●
High-throughput screening and purification of antibodies
Using Protein L Magnetic beads to purify the antibody from the serum as an example, the general purification protocol is as follows:
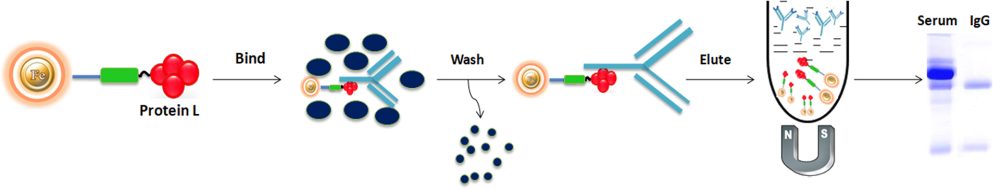
1.
Materials Required
a.
Buffer Composition
●
BcMag™ Protein L Beads (10 mM Tris, 0.15 M NaCl, 0.1% BSA,1 mM EDTA, pH 7.4, 0.1% NaN3)
●
1x Protein L Binding/Washing Buffer (0.1 M Sodium Phosphate, pH 7.0, 0.15 M NaCl)
●
1x Protein L Elution Buffer (0.2 M Glycine/HCl, pH 2.5)
●
1x Protein L Neutralization Buffer (1.0 M Tris-HCl, pH 9.0)
b.
Equipment
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
For larger scale purification, Ceramic magnets Block for large scale purification ( 6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8)
●
Corning 430825 cell culture flask for large-scale purification (Cole-Parmer, Cat. No. EW-01936-22)
●
Mini BlotBoy 3D Rocker, fixed speed, small 10″ x 7.5″ platform w/ flat mat (Benchmark Scientific, Inc. Cat. No. B3D1008) or compatible
2.
Procedure
Note:
1.
This protocol is optimized for purifying most IgG antibodies from different sources. However, designing a universal kit for all IgG purification is impossible because no two antibodies are identical. To obtain the best results, each user should determine the optimal working conditions for the purification of individual antibodies, especially for those weakly-binding antibodies (see Table 1).
2.
It is necessary to dilute serum samples, ascites fluid, or tissue culture at least 1:1 with Binding/Washing buffer before the purification.
3.
A.
Magnetic Beads Preparation
1.
Vigorously shake the bottle until the magnetic beads become homogeneous and transfer an appropriate volume of the magnetic beads to a new tube or flask.
Note:
●
Optimize the number of beads used for each application. Too many beads will cause higher background. Insufficient beads will lead to lower yields. We recommend 100 μl of the wholly suspended beads per ~55μg of IgG antibodies. Typically, a high-titer antiserum has roughly 5 mg/ml of IgG for rabbits, 10 mg/ml of IgG for Mouse ascites, and 20 mg/ml of IgG for goat or sheep antiserum.
●
Do not allow the beads to sit for more than 5 minutes before dispensing. Resuspend the magnetic beads every 3 minutes.
2.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Add ten bead-bed volumes of 1x Binding/Washing Buffer and mix the beads by pipetting or vortex. Place the tube on the magnetic rack for 1-3 minutes and remove the supernatant while the tube remains on the rack.
3.
Repeat step (2) one more time. The beads are ready for purification.
B.
Purification
1.
Add an equal volume of 1x Binding/Washing Buffer to the sample and mix well.
2.
Mix the equilibrated beads [Step A (3)] to the sample and incubate on Mini BlotBoy 3D Rocker with continuous rotation for 5-10 minutes at room temperature or 30-45 minutes at 4ºC.
3.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Add 10 bead-bed volumes of 1x Binding/Washing Buffer and shake it 10 times to wash the beads. Place the tube on the magnetic rack for 1-3 minutes and remove the supernatant while the tube remains on the rack.
4.
Repeat step (3) six times.
Note:
●
This step is critical to get high pure protein. It may be necessary to wash the beads more than six times for some proteins to reduce the nonspecific binding.
●
Add a nonionic detergent (0.5% Triton X 100, 0.5% Tween 20) to the binding/washing buffer, which may reduce the nonspecific binding.
5.
Add an appropriate amount of Elution Buffer and mix well by pipetting up and down 10-12 times or vortex mixer for 5 minutes.
6.
Collect and transfer the antibody-containing supernatant to a new tube. Immediately neutralize the eluted antibody solution by adding 1/10th volume of neutralization buffer and mix well.
1.
Sometimes, why could IgG not be eluted from the magnetic beads?
●
The pH of the Elution Buffer may be incorrect. The correct pH should be 2.5.
●
The elution conditions are too mild to elute the antibody.
●
Because a few antibodies can only be eluted at pH 2.0.
2.
What accounts for lost or decreased immuno-reactivity of the eluted antibody?
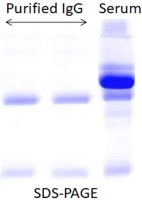
3.
Why are multiple bands observed in the eluted antibody solution?
Some host proteins may nonspecifically interact with your target antibody. Users can add NaCl (50-200mM, final concentration) to the binding and elution buffers.
A.
Additional Materials Required
●
1.5mL microcentrifuge tubes
●
Antibody for immunoprecipitation
●
Antigen sample
●
Lysis Buffer: 20 mM Tris HCl pH 8,137 mM NaCl, 1% Nonidet P-40 (NP-40), 2 mM EDTA. Store up to 6 months at 4°C. Immediately before use add protease inhibitor.
●
Wash buffers: 10mM Tris, pH 7.4, 1mM EDTA,1mM EGTA; pH 8.0, 0.5 M NaCl,1% Triton X-100, 0.05% Tween-20 0.2mM sodium orthovanadate, Protease inhibitor cocktail. Store up to 6 months at 4°C. Immediately before use add protease inhibitor. 0.05% Tween-20 Detergent and 0.5M NaCl.
●
Elution Buffer (0.2 M Glycine/HCl, pH 2.5)
●
Neutralization Buffer (1.0 M Tris-HCl, pH 9.0)
B.
Magnetic rack
●
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
C.
Immunoprecipitation procedure
Note: This protocol is an example of immunoprecipitation and will require optimization for each application.
1.
Sample preparation
a.
Adherent cells
a)
Place the cell culture dish or flask on ice, aspirate media, and wash the cells with cold PBS.
b)
Aspirate PBS, then add appropriate volume ice cold lysis buffer (1 mL per 107 cells/100 mm2 dish/150 cm2 flask; 0.5 ml per 5×106 cells/60 mm2 dish or 75 cm2 flask)
Note: Optimizing the volume of lysis buffer is necessary to ensure complete lysis and an optimal final concentration of protein in the lysate.
c)
Incubate for 20 minutes on ice and then Scrape adherent cells off the dish or flask using a cell scraper and transfer the cell suspension into a microfuge tube and clarify the lysate by spinning for 10 minutes at 13,000 rpm, at 4°C.
d)
Collect the supernatant (avoiding the pellet) into a new microfuge tube. Determine protein concentration by appropriate methods.
e)
Proceed with the immunoprecipitation or Store at -20°C or -80°C until needed.
b.
Suspension Cells
a)
Transfer cells into a conical tube.
b)
Spin to pellet cells at low speed at 1400 rpm for 5 minutes at room temperature and aspirate off media.
c)
Wash pellet with 10 ml ice-cold PBS.
d)
Spin cells on low speed (at 1400 rpm), and Decant the PBS wash.
e)
Repeat wash and aspiration.
f)
Add an appropriate volume of ice-cold lysis buffer (10 to 100 μl per 2 x 106 cells) and Incubate cells for 30 minutes on ice.
Note: If necessary, sonicate the lysates on ice for 20-30 seconds to shear genomic DNA and cellular components.
g)
Centrifuge at 13,000 rpm for 10 minutes at 4ºC.
h)
Collect the supernatant into a fresh tube and determine protein concentration by appropriate methods. Adjust concentration to 2.5 mg/ml with lysis buffer.
i)
Proceed with the immunoprecipitation or Store at -20°C or -80°C until needed.
c.
Lysates from tissue
a)
Chop the tissue into small pieces, and wash twice with ice-cold PBS.
b)
Transfer ~5 mg chopped tissue into a tube, and add 300μl cold lysis buffer. Homogenize with an electric homogenizer or sonicate on ice.
c)
Spin at 14000 rpm for 10 min at 4°C.
d)
Collect the supernatant into a fresh tube. Determine protein concentration by appropriate methods.
e)
Proceed with the immunoprecipitation or Store at -20°C or -80°C until needed.
2.
Note: Optimizing the amount of antibodies used for each application is necessary. Excess antibodies will cause higher background. Insufficient antibodies will lead to lower yields. Performing a pilot experiment is required to determine the optimum amount of antibody titer for maximum binding to the protein of interest. Typically in such an experiment, increasing amounts of antibodies for a fixed amount of protein is performed to establish the optimum titer. The following is a general guideline for antibody use. For individual antibodies, check the antibody instruction sheet for recommended antibody concentration.
For 100–150 μg cell lysate, use:
●
1–5 μL polyclonal antiserum
●
1 μg affinity-purified polyclonal antibody
●
0.2–1 μL ascites fluid (monoclonal antibody)
●
20–100 μL culture supernatant (monoclonal antibody)
3.
Transfer the appropriate amount of BcMag™ Protein L Magnetic Beads into a 1.5mL microfuge.
Note:
●
Vigorously shake the bottle until the magnetic beads become homogeneous before use.
●
The amount of the beads for use can be determined by the beads’ antibody binding capacity (~ 60μg IgG/mg ) and the antibody used.
●
Do not allow the beads to sit for more than 5 minutes before dispensing. Resuspend the magnetic beads every 3 minutes.
4.
Wash the beads with 1ml of Wash Buffer and gently vortex to mix. Collect the beads by a magnet and remove and discard the supernatant.
5.
Add the antigen sample/antibody mixture to the pre-washed magnetic beads and incubate at room temperature for 1 hour with continuous rotation.
6.
Collect the beads by a magnet, remove the supernatant, and save them for analysis.
7.
Wash the beads with 500μL of Wash Buffer by pipetting up and down 10 times. Collect the beads and discard the supernatant.
8.
Repeat wash twice until the absorbance of the supernatants at 280 nm approaches the background level (OD 280 < 0.05).
Note:
Adding a higher concentration of salts, nonionic detergent, and reducing agents may reduce the nonspecific background. For example, we are adding NaCl (up to 1-1.5 M), 0.1-0.5% nonionic detergents such as Triton X 100 or Tween 20, and a reducing reagent such as DTT or TCEP (usually 3mM) to the washing buffer.
9.
Add 500μL of ultrapure water to the tube and gently mix. Collect the beads on a magnetic stand and discard the supernatant.
10.
Troubleshooting
Problem
The yield of the purified protein is too low or undetectable in eluted protein solution by SDS-PAGE.
Probable Cause
The protein degraded or unstable
Suggestion
Add protease inhibitors.
Problem
The yield of the purified protein is too low or undetectable in eluted protein solution by SDS-PAGE.
Probable Cause
Not enough magnetic beads were used.
Suggestion
Increase the amount of magnetic bead used for capture.
Problem
The yield of the purified protein is too low or undetectable in eluted protein solution by SDS-PAGE.
Probable Cause
The protein does not bind to the Magnetic Beads.
Suggestion
Check the pH of all the buffers and solutions.
Problem
The yield of the purified protein is too low or undetectable in eluted protein solution by SDS-PAGE.
Probable Cause
The sample had an insufficient amount of target protein
Suggestion
Increase the amount of antigen sample.
Problem
The yield of the purified protein is too low or undetectable in eluted protein solution by SDS-PAGE.
Probable Cause
The protein is not efficiently eluted from beads.
Suggestion
Increase incubation time with elution buffer.
Problem
Observe multiple bands in the eluted protein
Probable Cause
Nonspecific protein bound to the magnetic beads
Suggestion
Increase NaCl to the Binding/Wash Buffers.
Problem
Observe multiple bands in the eluted protein
Probable Cause
Degradation of the antigen
Suggestion
Add appropriate protease inhibitor.
Problem
Observe multiple bands in the eluted protein
Probable Cause
The washing condition is not optimized.
Suggestion
Problem
Probable Causes
Suggestions
The yield of the purified protein is too low or undetectable in eluted protein solution by SDS-PAGE.
The protein degraded or unstable
Add protease inhibitors.
Not enough magnetic beads were used.
Increase the amount of magnetic bead used for capture.
The protein does not bind to the Magnetic Beads.
Check the pH of all the buffers and solutions.
The sample had an insufficient amount of target protein
Increase the amount of antigen sample.
The protein is not efficiently eluted from beads.
Increase incubation time with elution buffer.
Observe multiple bands in the eluted protein
Nonspecific protein bound to the magnetic beads
Increase NaCl to the Binding/Wash Buffers.
Degradation of the antigen
Add appropriate protease inhibitor.
The washing condition is not optimized.
Magnetic Beads Make Things Simple