- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]
Products
Specification
Composition
Magnetic beads grafted with quaternary amine functional groups.
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.0 g/ml
Stability
Most organic solvents
Strong Anion Exchange beads
1 μm beads: ~3 mg BSA/ ml of Beads
5 μm beads: ~2 mg BSA/ ml of Beads
Storage
Store at 4°C upon receipt.
Magnetic Beads fractionate proteins or nucleic acids using beads-adsorbent technology as a chromatographic matrix. Ion exchange chromatography is widely used to separate or purify a target molecule from crude biological materials. The molecules are separated based on variations in their accessible surface charges utilizing very light binding and eluting conditions for intact biological activity.
BcMag™ Strong Anion Exchange (SAX) Magnetic Beads are uniform magnetic resins grafted with a high density of quaternary amine functional group (Strong Anion Exchange) on the surface. The strong anion exchange magnetic bead-based format enables rapid high-yield processing of 96 samples in about 20 minutes. It can quickly fraction proteins or nucleic acids from complex biological samples (such as serum, plasma, etc.) manually or automatically. The purified protein can be used in downstream applications such as sample fractionation for 1D and 2D SDS-PAGE, X-ray crystallization, and NMR spectroscopy. Additionally, Strong ion exchangers can be effective separation tools when weak ion exchangers fail because the selectivity of weak and strong ion exchangers frequently differ.
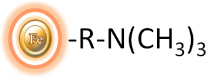
Strong anion exchange magnetic resins are used to replace time-consuming, complex, and costly chromatographic procedures such as agarose, cellulose, Sepharose, and Sephadex-based columns or resins. In column-based procedures, the lysate is centrifuged or cleared, the supernatant is added to the column, the membrane or resin is washed with buffer through centrifugation or vacuum manifold, and the required biomolecules are eluted in an adequate volume of buffer. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in target biomolecule yield between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields.
Magnetic resins have significant advantages over non-magnetic resin technologies. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high throughput automated and miniaturized processing. They thus see increasing use in various areas of life-sciences research and development, including drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics.
●
Fast and simple – Magnetic beads-based format eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Convenient and expandable – Magnetic format enables high-throughput processing of multiple samples in parallel with many different automated liquid handling systems.
●
Robust – Magnetic beads do not crack or run dry.
●
Low bed volume – Working with small magnetic bead volumes allows for minimal buffer volumes, resulting in concentrated elution fractions.
●
Protein pre-fractionation in cell lysates
●
Optimizing purification conditions for new protein preparation protocols
●
Protein purification and concentration
●
Antibody purification from serum, ascites, or tissue culture supernatant
●
Preparation of samples before 1D or 2D PAGE
●
Phosphopeptide purification before MS analysis
1.
Wang, F.; Han, G.; Yu, Z.; Jiang, X.; Sun, S.; Chen, R.; Ye, M.; Zou, H. Fractionation of phosphopeptides on strong anion-exchange capillary trap column for large-scale phosphoproteome analysis of microgram samples J. Sep. Sci. 2010, 33 (13) 1879– 87
2.
Alpert AJ, Hudecz O, Mechtler K (2015) Anion-exchange chromatography of phosphopeptides: weak anion exchange versus strong anion exchange and anion-exchange chromatography versus electrostatic repulsion-hydrophilic interaction chromatography. Anal Chem 87: 4704–4711
3.
Han G, Ye M, Zhou H, Jiang X, Feng S, Jiang X, Tian R, Wan D, Zou H, Gu J (2008) Large-scale phosphoproteome analysis of human liver tissue by enrichment and fractionation of phosphopeptides with strong anion exchange chromatography. Proteomics 8: 1346–1361
4.
Liang X, Hu P, Zhang H, Tan W. Hypercrosslinked strong anion-exchange polymers for selective extraction of fluoroquinolones in milk samples. J Pharm Biomed Anal. 2019 Mar 20;166:379-386.
5.
Laha D, Kamleitner M, Johnen P, Schaaf G. Analyses of Inositol Phosphates and Phosphoinositides by Strong Anion Exchange (SAX)-HPLC. Methods Mol Biol. 2021;2295:365-378.
6.
Gu F, Chodavarapu K, McCreary D, Plitt TA, Tamoria E, Ni M, Burnham JJ, Peters M, Lenhoff AM. Silica-based strong anion exchange media for protein purification. J Chromatogr A. 2015 Jan 9;1376:53-63.
7.
Ritorto MS, Trost M, Cook K. Hydrophilic strong anion exchange chromatography for proteomics: what’s the future outlook? Bioanalysis. 2013 Sep;5(18):2219-21.
Magnetic Beads Make Things Simple