- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]
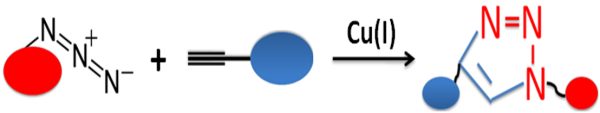
The copper-catalyzed azide-alkyne cycloaddition (CuAAC) is an efficient and reliable reaction that uses the copper-catalyzed coupling of azides to terminal acetylenes via powerful linking (1,4-disubstituted triazole) to produce unique practical and versatile new biological compounds. (Fig.1). CuAAC, the most used click chemistry, is widely used in bioconjugation, drug discovery, materials science, and radiochemistry due to its numerous unique advantages.
1.
Fast kinetics and high efficiency
The tetrazine cycloaddition reaction is extremely fast and takes only minutes to complete. Due to such a high reaction rate, it works well even with an ultra-low concentration of reagents for most biological systems. Moreover, the reaction produces minimal byproducts but quantitative yields. Rapid and quantitative labeling allows non-radioactive analysis of enzymatic activities both in vitro and in vivo: Small-sized CLICK-functional groups possess excellent substrate properties.
2.
Outstanding selectivity and specificity
The azide or alkynes moiety is absent in almost all native biomolecules, and the reaction occurs only between terminal alkynes and azides groups. It is performed in the presence of other functional moieties, which allow selective ligation with a very limited set of reaction partners.
3.
Very flexibility
The small size and remarkable stability of azide, alkyne, or linkage (1,4-disubstituted triazole) are proven to be exceptionally suitable for relatively easy incorporation into a variety of molecules such as synthetic polymers, fluorophores, small molecules, or into specific locations in biomolecules, that imposes minimal perturbation to the structure and function of the conjugated biomolecules.
4.
Mild reaction condition
The reaction can be performed under various conditions, including an organic solvent, pure water, buffers, a wide pH (pH 4-11), and temperature range.
We provide Alkyne and Azide-activated magnetic beads-based systems as more efficient tools to meet the increasingly demanding applications in using azide-alkyne click reactions.
Table – Alkyne and Azide-activated magnetic beads
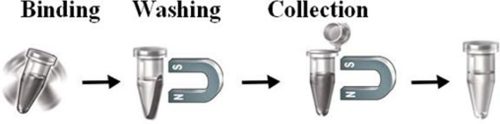
The activated magnetic beads are uniform inert silica-based magnetic beads grafted with a high density of alkyne or azide functional groups on the surface. The beads are designed to efficiently enrich alkyne or azide-tagged biomolecules from complex cell lysates via a Cu(I)-catalyzed Alkyne-Azide (CUAAC) reaction. Compared with other affinity resins such as agarose beads or other polymers, the inert silica-enclosed magnetic beads offer high stability, low nonspecific binding, and superior handling in protein-based systems. Since the active cleavable alkyne or azide group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads after affinity purification. These magnetic beads are ideal tools for genomics, proteomics, biomarker discovery, posttranslational modification (PTM) analysis, etc.
The beads work perfectly as affinity resin for capturing alkyne- or azide- tagged biomolecules from complex cell lysate. Add the beads to a sample containing the tagged biomolecules, then mix, incubate, wash and elute the target molecules
1.
A.V. Ustinov, I.A. Stepanova, V.V. Dubnyakova, T.S. Zatsepin, E.V. Nozhevnikova, V.A. Korshun. Modification of nucleic acids using [3+2]-dipolar cycloaddition of azides and alkynes. Russ. J. Bioorg. Chem. 36(4), 401–445 (2010).
2.
A.H. El-Sagheer, T. Brown. Click chemistry with DNA. Chem. Soc. Rev. 39(4), 1388–1405 (2010).
3.
F. Amblard, J.H. Cho, R.F. Schinazi. Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 109(9), 4207–4220 (2009).
4.
Franck Amblard, Jong Hyun Cho, and Raymond F. Schinazi; The Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem Rev. 2009 September; 109(9): 4207–4220. doi:10.1021/cr9001462.
5.
Z. P. Demko and K. B. Sharpless, An Expedient Route to the Tetrazole Analogs of α–Amino Acids, Org. Lett., 4, 2525 (2002).
6.
Hartmuth C. Kolb, M. G. Finn, and K. Barry Sharpless; Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004 ± 2021.
Magnetic Beads Make Things Simple