- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Bioclone Strong Ion Exchange Magnetic Beads fractionate proteins or nucleic acids using beads-adsorbent technology as a chromatographic matrix. Strong Ion exchange chromatography is commonly used to separate or purify a target molecule from crude biological materials. Molecules are usually separated using very mild binding and eluting conditions based on changes in their accessible surface charges, allowing high biomolecule recovery with intact biological activity.
The Strong Ion Exchange magnetic resins replace time-consuming, difficult, and expensive chromatographic techniques such as agarose, cellulose, Sepharose, and Sephadex-based columns or resins. In column-based methods, the lysate would be centrifuged or clarified, the supernatant would be added to column, the membrane or resin would be washed with buffer via centrifugation or vacuum manifold, and the desired biomolecules would be eluted in an appropriate volume of buffer. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can result in discouraging differences in target biomolecule yield between experiments and people. To produce reasonably constant nucleic acid yields, staff and students may require extensive training and practice.
Magnetic resins have significant advantages over non-magnetic resin technologies. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high-throughput automated and miniaturized processing. They thus see increasing use in various areas of life-sciences research and development, including drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics.
Ion exchange chromatography (IEX) separates ionizable compounds according to their total charge. Because adjusting buffer pH can easily change the charge on the molecule, this technique allows for separating similar types of molecules that would be hard to separate using other methods. Ion exchange chromatography is a highly effective separation technology for preparative and analytical chromatography. It is widely used to separate and purify many charged or ionizable molecules such as proteins, peptides, enzymes, nucleotides, DNA, antibiotics, vitamins, etc.
BcMag™ Strong Ion Exchange (SAX) Magnetic Beads are uniform magnetic resins grafted with a high density of quaternary amine functional group (Strong Anion Exchange) or strong cation exchanger (sulphonic acid functional groups) on the surface. The magnetic bead-based format enables rapid high-yield processing of 96 samples in about 20 minutes. It can quickly fraction proteins or nucleic acids from complex biological samples (such as serum, plasma, etc.) manually or automatically. The purified protein can be used in downstream applications such as sample fractionation for 1D and 2D SDS-PAGE, X-ray crystallization, and NMR spectroscopy. Additionally, Strong ion exchangers can be effective separation tools when weak ion exchangers fail because the selectivity of weak and strong ion exchangers frequently differ.
●
Fast and simple – Magnetic beads-based format eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Convenient and expandable – Magnetic format enables high-throughput processing of multiple samples in parallel with many different automated liquid handling systems.
●
Robust – Magnetic beads do not crack or run dry.
●
Low bed volume – Working with small magnetic bead volumes allows for minimal buffer volumes, resulting in concentrated elution fractions.
●
Protein pre-fractionation in cell lysates
●
Optimizing purification conditions for new protein preparation protocols
●
Protein purification and concentration
●
Antibody purification from serum, ascites, or tissue culture supernatant
●
Preparation of samples before 1D or 2D PAGE
●
Phosphopeptide purification before MS analysis
●
For the convenience of usage, each Bioclone Strong Ion Exchange Bead is labeled SCX (strong cation exchanger, sulphonic acid functional groups) or SAX. (strong anion exchanger, quaternary ammonium functional groups) or PEI (quaternized branched polyethyleneimine groups). Table 1 lists the pKa of Strong Ion exchange beads.
Table 1 – pKa of Strong Ion Exchange Beads
Beads
pKa
Strong Anion Exchange
Magnetic Beads
A Strong Anion Exchange (SAX) Beads, pKa =~12
A Strong Anion Exchange (PEI) Beads, pKa =~10
Strong Cation Exchange
Magnetic Beads
A Strong Cation Exchange (SCX) Beads, pKa =~2.3
●
The magnetic bead binding capacities: >80 µg Lysozyme/ mg of Beads for SCX assayed in 25 mM sodium acetate buffer, pH 5.5, and 70-80 BSA / mg of Beads for SAX determined in 25 mM Tris•HCl, pH 8.0.
●
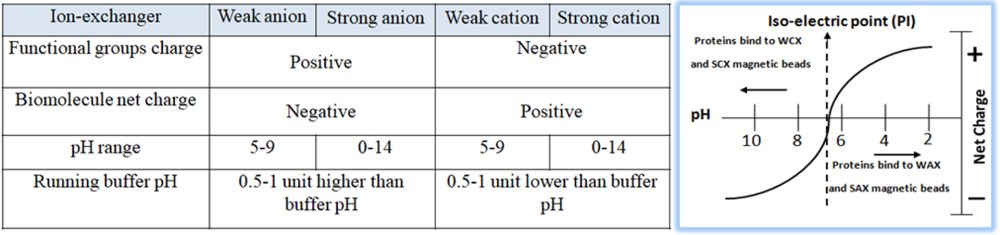
Based on the protein’s pI, empirically calculate the appropriate buffer (pH and salt concentration) for purifying and eluting the protein of interest. In a buffered solution above the protein’s pI, the protein becomes negatively charged (deprotonated) and binds to the positively charged functional groups of an anion exchange resin. To choose the correct buffer for a selected pH, the following is a general rule for selecting a buffer pH:
Anion exchanger — 0.5–1.5 pH units higher than the protein’s pI of interest.
Cation exchanger — 0.5–1.5 pH units lower than the protein’s pI of interest.
●
For the anion resins, ensure that the buffer pH is greater than the protein’s isoelectric point and that the sample does not contain anionic detergents. Use a buffer with a pH of 5 to 10 and a salt concentration of ≤ 25 mM to purify the sample. Use a stepwise salt gradient to achieve a final salt concentration of 2 M for elution.
●
For the cation resins, ensure that the buffer pH is less than the protein’s isoelectric point and that the sample contains no cationic detergents. Use a buffer with a pH range of 3 to 8 and a salt content of ≤ 25 mM to purify the sample. Use a stepwise salt gradient to achieve a final salt concentration of 2 M for elution.
●
For successful purification, it is necessary to dilute, dialysis, and gel filtration of the protein sample before mixing it with the magnetic beads. Dilute the material with buffer ≤ 25 mM salt for the best results.
Specification
Composition
Silica-coated iron oxide grafted with a strong ion exchange group.
Beads Size
~1.0 µm diameter
~5.0 µm diameter
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.0 g/ml
Stability
Most organic solvents
Binding Capacity
SAX Beads
1 µm beads: ~80 µg BSA/ mg of Beads
5 µm beads: ~70 µg BSA/ mg of Beads
PEI Beads
1 µm beads: ~85 µg BSA/ mg of Beads
5 µm beads: ~75 µg BSA/ mg of Beads
SCX Beads
1.0 µm beads: >70 µg Lysozyme / mg of Beads
5 µm beads: >55 µg Lysozyme / mg of Beads
Storage
Store at 4°C upon receipt
a. Equipment
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
For larger scale purification, Ceramic Magnets Block for large scale purification (6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8).
●
Corning 430825 cell culture flask for large-scale purification (Cole-Parmer, Cat. No. EW-01936-22)
●
Mini BlotBoy 3D Rocker, fixed speed, small 10″ x 7.5″ platform w/ flat mat (Benchmark Scientific, Inc. Cat. No. B3D1008) or compatible
b. Sample
●
Binding/Washing buffer: The pH of the Binding/Washing Buffer should be at least one pH unit away from the pI of the target protein or peptide. (Note: For best results, the salt should be ≤ 25mM in the sample). If necessary, dilute the sample with purification buffer to minimize its ionic strength. For purifying Basic Proteins (high pI) with Strong Cation Magnetic Resins, for example, Binding/Washing buffer (25 mM sodium acetate buffer, pH 5.5). For purifying Acidic Proteins (low pI) with Strong Anion Magnetic resins, for example, Binding/Washing buffer (25 mM Tris•HCl buffer, pH 8.0).
●
Elution Buffer: To elute the target protein or peptide from the magnetic resins, the user should optimize elution condition for individual application by stepwise elution either using solutions with increasing salt concentration, i.e., increase stepwise to a final salt concentration of 2.5 M) or changing the pH of the elution buffer. For purifying Basic Proteins (high pI) with Strong Cation Magnetic Resins, for example, Elution buffer (25 mM sodium acetate buffer, pH 5.5 containing 0.5 or 1.0 M NaCl). For purifying Acidic Proteins (low pI) with Strong Anion Magnetic resins, for example, Elution buffer (25 mM Tris•HCl buffer, pH 8.0 containing 0.5 or 1.0 M NaCl).
A. General Protocol for using the Magnetic Beads –
a.
Magnetic beads preparation
1.
Vigorously shake the bottle until the magnetic beads become homogeneous and transfer an appropriate volume of the magnetic beads from the bottle to a new tube or flask.
Note:
●
Optimize the number of beads used for each application. Too many beads will cause higher background. Insufficient beads will lead to lower yields.
●
2.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Add ten bead-bed volumes of dH2O2 and mix the beads by pipetting or vortex. Again, place the tube on the magnetic rack for 1-3 minutes and remove the supernatant while the tube remains on the rack.
3.
Repeat step (2) one more time.
4.
Equilibrate the beads by adding ten bead-bed volumes of Binding/Washing buffer and shake it to mix them. Incubate at room temperature with continuous rotation for 2 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. The beads are ready for purification.
b.
Purification
1.
Add the equilibrated beads (Step a (4)) to the sample and incubate on Mini BlotBoy 3D Rocker with continuous rotation for 5-10 minutes.
2.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Add ten bead-bed volumes of Binding/Washing buffer and shake it ten times to wash the beads. Again, place the tube on the magnetic rack for 1-3 minutes and remove the supernatant while the tube remains on the rack.
3.
Repeat step (2) six times.
Note:
●
This step is critical to get high pure protein. It may be necessary to wash the beads more than six times for some proteins to reduce the nonspecific binding.
●
Optimize the washing buffer (pH and salt concentration)
Elute protein with an appropriate volume of elution buffer by pipetting up and down 10-15 times or vortex mixer for 5 minutes.
Note:
Determine the optimum elution buffers (pH and salt concentration) and eluting the protein 2-3 times may be necessary.
4.
Elute protein with an appropriate volume of elution buffer by pipetting up and down 10-15 times or vortex mixer for 5 minutes.
Note:
Determine the optimum elution buffers (pH and salt concentration) and eluting the protein 2-3 times may be necessary.
5.
Collect and transfer the supernatant to a new tube.
B. Troubleshooting
Problem
Low yield
Probable Cause
The sample’s ionic strength is high.
Suggestion
Problem
Low yield
Probable Cause
The sample contains interfering detergents.
Suggestion
Problem
The protein failed to elute.
Probable Cause
Ionic interaction is too strong.
Suggestion
Problem
Poor separation
Probable Cause
Carry-over between eluted fractions
Suggestion
Problem
Poor separation
Probable Cause
Proteins or peptides with similar pI to the target protein
Suggestion
Problem
Probable Cause
Suggestions
Low yield
The sample’s ionic strength is high.
The sample contains interfering detergents.
The protein failed to elute.
Ionic interaction is too strong.
Poor separation
Carry-over between eluted fractions
Proteins or peptides with similar pI to the target protein
1.
Huong DTM, Liu BL, Chai WS, Show PL, Tsai SL, Chang YK. Highly efficient dye removal and lysozyme purification using strong and weak cation-exchange nanofiber membranes. Int J Biol Macromol. 2020 Dec 15;165(Pt A):1410-1421
2.
Winnik WM. Continuous pH/salt gradient and peptide score for strong cation exchange chromatography in 2D-nano-LC/MS/MS peptide identification for proteomics. Anal Chem. 2005 Aug 1;77(15):4991-8.
3.
Wang NW. Ion exchange in purification. Bioprocess Technol. 1990;9:359-400
4.
Wittkopp F, Peeck L, Hafner M, Frech C. Modeling and simulation of protein elution in linear pH and salt gradients on weak, strong and mixed cation exchange resins applying an extended Donnan ion exchange model. J Chromatogr A. 2018 Apr 13;1545:32-47.
5.
Toribio A, Nuzillard JM, Renault JH. Strong ion-exchange centrifugal partition chromatography as an efficient method for the large-scale purification of glucosinolates. J Chromatogr A. 2007 Nov 2;1170(1-2):44-51.
6.
Edelmann MJ. Strong cation exchange chromatography in analysis of posttranslational modifications: innovations and perspectives. J Biomed Biotechnol. 2011;2011:936508.
Magnetic Beads Make Things Simple