- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]
Samples for bioassays are regularly prepared in aqueous suspensions of various body fluids, which may contain a variety of nonvolatile salts, solvents, albumins, immune globulins, and lipids. These contaminants or matrix proteins can affect the performance of analytical methods, reducing specificity and sensitivity by interfering with adduct formation, precipitation, or modification. Additionally, the most often utilized analytes are nucleic acids or proteins. They can be abundant in the primary damaged organ, but they become diluted when they spread throughout the body, as in the case of cancer. The ability to identify the low concentration of analytes allows for successful bioassays. Enzyme-linked immunosorbent assays (ELISA) are the most extensively used method for protein analysis in fundamental research and clinical diagnostics. However, ELISA is extremely slow, requiring many reagent and washing steps that are both time-consuming and labor-intensive and not ideal for high-throughput assays with small assay volumes. TR-FRET (Time-Resolved FRET) is a well-known technology widely used in clinical diagnostics and academic laboratories. TR-FRET has proven to be a highly versatile assay approach, allowing researchers to study a wide range of biological interactions with low to high affinity, using both small and large molecules.
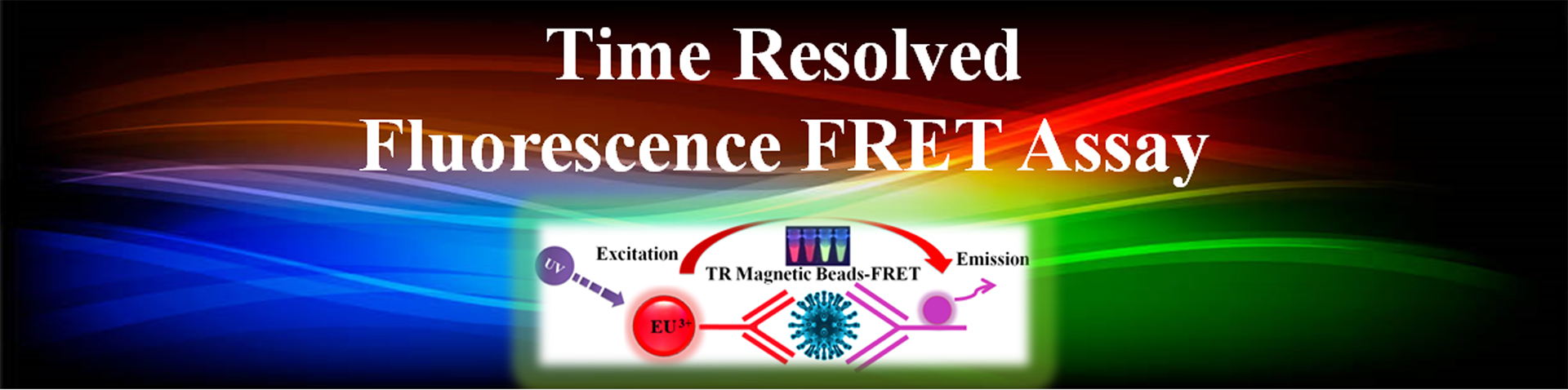
Förster resonance energy transfer (FRET) is based on the transfer of energy between two fluorophores in close proximity, a donor (long-lived fluorescence) and an acceptor (short-lived fluorescence). The level of energy transfer between biomolecules can be measured by labeling each partner with a Fluorescence marker and measuring the level of interaction. Organic luminous chemicals such as fluorescein and rhodamine were once commonly utilized in fluorescence assays. However, these bioassays have significant disadvantages in that Fluorescence detection. Because it is significantly inhibited by noise in the background derived from scattered excitation light and seriously interfered with by fluorescence from coexisting material in the sample (Fluorescence compounds and dust/line), obtaining a susceptible measurement is difficult.
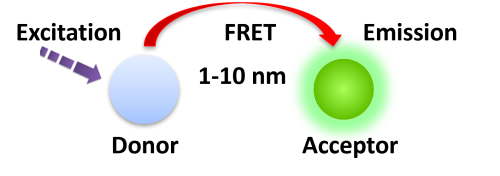
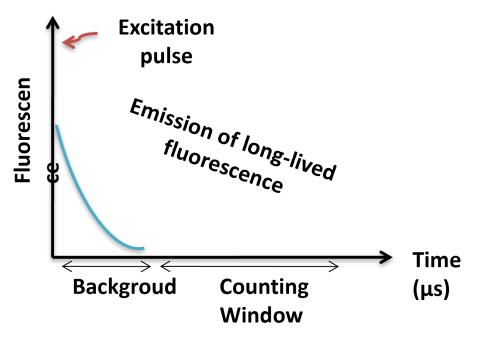
TR-FRET combines time-resolved fluorescence measurement (TR) with fluorescence resonance energy transfer (FRET) technology. Biomolecules are labeled with donor and acceptor fluorophores in FRET assays. Donor and acceptor fluorophores are brought close together when the biomolecules interact. When excited, the donor can transfer its emission energy to the acceptor, which emits fluorescence at a specified wavelength. The wavelengths of acceptor and donor fluorescence emissions differ and can be identified using a microplate reader, allowing quantification of the biomolecular interaction.
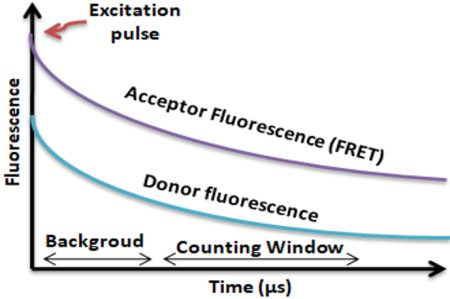
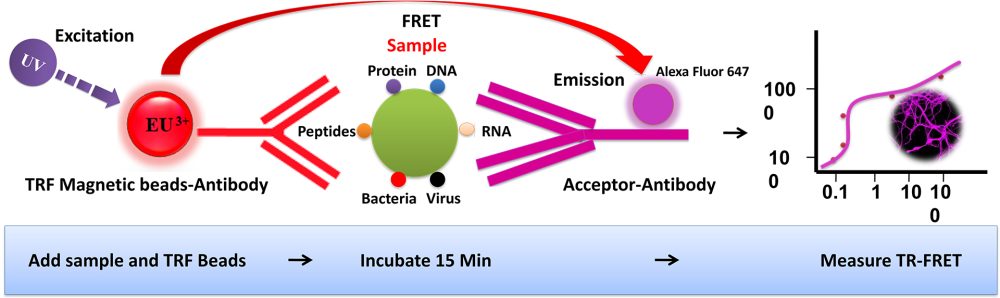
BcMag™ TR-FRET Assay, in contrast to typical FRET assays, uses time-resolved Fluorescence magnetic beads (BcMag™ TR-Magnetic Beads) as the donor fluorophore. The donor and acceptor can be two proteins, two DNA strands, an antigen, an antibody, or a ligand and its receptor. After a reasonable time delay (usually 50 to 100 s), a signal is generated by fluorescence resonance energy transfer between a donor and an acceptor molecule when they are close and monitored in a time-resolved way. In BcMag™ TR-FRET Assay, a trace amount of analytes can be easily enriched from the complex by TR-Magnetic Beads, resulting in higher sensitivity. This assay practically eliminates all fluorescence backgrounds caused by the sample and plastic microplate, as well as by direct acceptor excitation. As a result, the signal-to-noise ratios of the BcMag™ TR-FRET Assay are very high, and the background is quite low. Furthermore, the assay does not need washing steps. BcMag™ TR-FRET Assay offers substantial advantages to bioassays in high throughput screening, such as assay flexibility, dependability, increased assay sensitivity, higher throughput, and fewer false positive/false negative results.
Magnetic bead separation is a fast, effective, and clean method used by scientists to replace filtering, centrifugation, and separation processes. Time-resolved Fluorescence Magnetic Beads can be used for immunoassays and other applications. They have high surface-to-volume ratios, small sizes (0.1-10µm), various functional groups attached to the surfaces (e.g., antibodies, DNA, and chemical groups), and the ability to manipulate the particles via an applied magnetic field easily. Combined with automated liquid handling and robust detection instrumentation, these characteristics enable a wide range of high-throughput applications.
Bioclone offers three unique Time-resolved Fluorescence Magnetic Beads: BcMag™ Europium Fluorescence Magnetic Beads, BcMag™ Terbium Fluorescence Magnetic Beads, and BcMag™ Ruthenium Fluorescence Magnetic Beads. They are uniform and monodisperse available in nominal diameters of 2.5µm and 5µm. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and Europium (Eu3+ cryptate) or Terbium (Tb3+ cryptate), or Ruthenium (Ru2+ cryptate) metal as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide and the metal. Their fluorescence properties are summarized in Table 1.
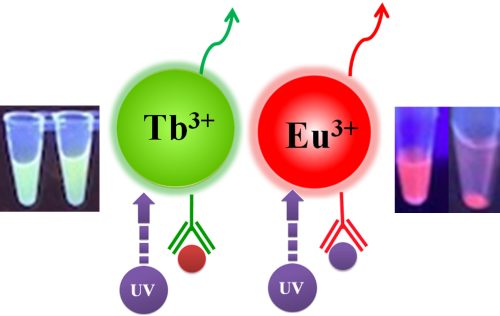
Table 1. Fluorescence Properties of TRF Magnetic Beads
Fluorophore
Red
Green
Far-Red
340
320
470
615
545
710
Fluorescence lifetime (Ԏ) (µsec)
730
1050
354.36
Stokes shifts
(nm)
275
220
175
Fluorophore
Fluorescence lifetime (Ԏ) (µsec)
Stokes shifts
(nm)
Red
340
615
730
275
Green
320
545
1050
220
Far-Red
470
710
354.36
175
1.
Perform a double function simultaneously on the same beads: The magnetic beads combine separation/preconcentration and detect analytes, allowing quick, simple, robust, and high-throughput analytes of trace amounts from complex biological samples on the same beads.
2.
Ultra sensitive. Lower detection limits of 10 pg/mL versus typical fluorometric detection limits of 100 pg/mL.
3.
Extremely photostable and highly resistant to photobleaching. All the lanthanides chelate or cryptate molecules and iron oxide are entirely encapsulated inside each bead instead of merely on the bead’s surface. The protective environment prevents iron oxide and dye from leaching into aqueous media, which makes the beads less sensitive to external conditions such as solvent, temperature, pH, etc.
4.
Very high Fluorescence intensity. Because a single bead has a large concentration of lanthanide chelate with a high quantum yield ranging from 40 to 90%, the beads show excellent fluorescence intensity, which increases test sensitivity without signal amplification. Such bright beads are also perfect for donors’ use in time-resolved FRET assays.
5.
Lanthanides chelate or cryptate has large Stokes shifts (>250 nm), narrow emission bands (-10 nm bandwidth), and long fluorescence lifetime (μs), which dramatically reduces background and increases the signal-to-noise ratio.
6.
Most bioprocess ELISA assays can be converted to an HTRF assay.
7.
No washing step is involved in the assays.
8.
Have a hydrophilic silica surface grafted by different functional groups with linkers of variable lengths, allowing efficient conjugation of various ligands such as peptides, proteins, antibodies, small molecules, carbohydrates, aptamers, DNA/RNA, etc.
9.
Due to the microsphere’s magnetic properties, the Fluorescence magnetic beads are suitable for high-throughput automation.
The TRF beads assay is straightforward.
1.
Mix the antibody-conjugated donor beads with the cell lysates and incubate them with continuous rotation for a sufficient time. The beads remain suspended in the sample solution during mixing, allowing the target analytes to bind to the donor beads.
2.
After incubation, the beads are collected and separated from the sample using a magnet rack.
3.
Add the antibody-conjugated acceptor and incubate them with continuous rotation for a sufficient time.
4.
Analysis of numerous microplate readers supports TR-FRET measurements.
Selection of TRF magnetic beads and acceptor
Table 2. Selection of TRF Magnetic Beads and Acceptor
TRF-Magnetic Beads
Excitation
Emission
Acceptor
BcMag™ Europium Fluorescence Magnetic Beads
340 nm
615 nm
BcMag™ Europium Fluorescence Magnetic Beads
340 nm
615 nm
AlexaFluor 647
BcMag™ Europium Fluorescence Magnetic Beads
340 nm
615 nm
XL665/d2
BcMag™ Terbium Fluorescence Magnetic Beads
320 nm
545 nm
Fluorescein / GFP
BcMag™ Ruthenium Fluorescence Magnetic Beads
470 nm
710 nm
Far-red Dye
Fluorescence Detection and Measurement
Fluorescence Detection and measurement can be used using the following instruments:
1.
Molecular Devices, LLC
●
●
The SpectraMax® iD5 Multimode Microplate Readers
2.
Thermo Fisher Scientific
●
Varioskan LUX Multimode Microplate Reader
3.
Medisensor, Inc.
●
Qcare™ TRF-S reader
4.
BioTek
●
Cytation 1 cell imaging multimode reader
5.
PerkinElmer
●
EnVision 2104-0020 Multilabel Microplate Reader Pred 2105
●
EnSpire 2300 MultiMode Microplate Reader
6.
BMG LABTECH
●
1.
Corr SA, Rakovich YP, Gun’ko YK. Multifunctional Magnetic-Fluorescence Nanocomposites for Biomedical Applications. Nanoscale Res Lett. 2008 Mar 6;3(3):87–104.
2.
Diamandis EP 1988. Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem. 1988 Jun;21(3):139-50.
3.
Fan W, Liu D, Ren W, Liu C. Trends of Bead Counting-Based Technologies Toward the Detection of Disease-Related Biomarkers. Front Chem. 2020 Dec 21;8:600317.
4.
Leng, Y., Sun, K., Chen, X., and Li, W. (2015). Suspension arrays based on nanoparticle-encoded microspheres for high-throughput multiplexed detection. Chem. Soc. Rev. 44, 5552–5595
5.
Ma, F., Li, Y., Tang, B., and Zhang, C. (2016). Fluorescence biosensors based on single-molecule counting. Acc. Chem. Res. 49, 1722–1730
6.
Parsa, S. F., Vafajoo, A., Rostami, A., Salarian, R., Rabiee, M., Rabiee, N., et al. (2018). Early diagnosis of disease using microbead array technology: a review. Anal. Chim. Acta 1032, 1–17.
7.
Werts, M. H. V. (2005). “Making Sense of Lanthanide Luminescence.” Science Progress. 88 (2): 101–131
8.
Rödiger, S., Liebsch, C., Schmidt, C. et al. Nucleic acid detection based on the use of microbeads: a review. Microchim Acta 181, 1151–1168 (2014).
9.
Soini, Erkki; Lövgren, Timo; Reimer, Charles B. (January 1987). “Time-Resolved Fluorescence of Lanthanide Probes and Applications in Biotechnology”. C R C Critical Reviews in Analytical Chemistry. 18 (2): 105–15
10.
Satoshi Sakamoto, Kenshi Omagari, Yoshinori Kita, Yusuke Mochizuki, Yasuyuki Naito, Shintaro Kawata, Sachiko Matsuda, Osamu Itano, Hiromitsu Jinno, Hiroya Takeuchi, Yuki Yamaguchi, Yuko Kitagawa, Hiroshi Handa, Magnetically Promoted Rapid Immunoreactions Using Functionalized Fluorescence Magnetic Beads: A Proof of Principle, Clinical Chemistry, Volume 60, Issue 4, 1 April 2014, Pages 610–620
Magnetic Beads Make Things Simple