- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Affinity chromatography, also known as bio-selective adsorption, makes use of specific binding affinity between two compounds. In this technology, a specific ligand is immobilized to an inert support matrix in such a way that when a sample is passed through it, molecules with specific affinity to the ligand connect to it. After washing away non-specific molecules in the sample, the bound molecule(s) can be easily eluted from the ligand linked to the matrix. Affinity chromatography uses a protein’s biological structure to purify it, resulting in high capacity, selectivity, and resolution. As a result, affinity chromatography is frequently used for simple purification.
Affinity separation ligands include:
●
Small chemical molecules have the ability to attach into protein binding sites
●
Inorganic metals that form coordination compounds with certain amino acids in proteins
●
Hydrophobic compounds that can bind nonpolar spaces in biomolecules
●
Proteins have particular binding sites that can interact with other proteins
●
Antibodies, which can be programmed to target any biomolecule via their antigen binding sites
The use of immobilized affinity ligands to purify biomolecules has evolved beyond chromatographic applications with beaded agarose resins (still the most common). Magnetic beads have significant advantages over non-magnetic bead technologies. They thus see increasing use in various areas of life-sciences research and development, including drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics. This is because magnetic beads have numerous advantages, including their ease of use, rapid experimental protocols, suitability and convenience for high-throughput automated and miniaturized processing, such as high-throughput screening, and the potential for scalability due to the availability of large homogenous biomagnetic separation equipment. Magnetic affinity separations have been used successfully in various domains, including molecular biology, biochemistry, immunochemistry, enzymology, analytical chemistry, and environmental chemistry.
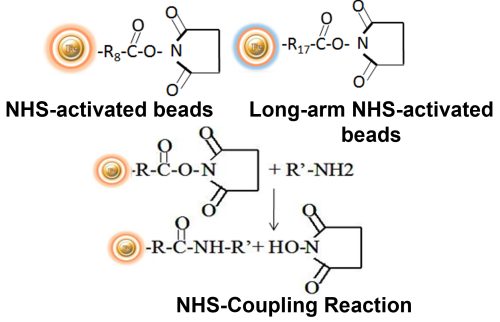
Making ligand-specific affinity magnetic beads requires methods to covalently conjugate ligands to appropriate magnetic beads. Immobilization and conjugation refer to the covalent attachment of ligands to magnetic beads through their common chemical groups. This method prepares the matrices by first activating with reactive chemical compounds toward one or more of these functional groups.
These chemical groups should be easily activated, such as primary amines, sulfhydryls, aldehydes, carboxylic acids, etc. The activated matrices then can conjugate antigens through a covalent linkage, resulting in ligand immobilization.
The type of linkage created between the matrix and the immobilized ligand influences the affinity support’s performance in a variety of ways. For example, if the linkage obstructs or negatively influences the structure of the immobilized ligand, its efficiency for affinity purification will be limited. A connection that allows the coupled ligand to leach from the matrix will contaminate the purified protein and reduce the affinity support’s useful life. Nonspecific binding can be caused by conjugation chemistry that puts a charged functional group into the support by enhancing ion-exchange effects. A connection that changes the matrix’s structure can modify the flow and binding properties of the support.
Affinity ligands can be linked (immobilized) to the solid substance (stationary phase) in a variety of ways.
The following general considerations apply for creating a successful magnetic affinity support matrix.
●
Linkers/spacers: Linkers are flexible molecules or stretch of molecules that link two molecules of interest together. The linkers’ property affects the affinity support’s performance in several ways.
●
• Linker length. Suppose ligands are small peptide antigens or molecular. In that case, it is best to use a long arm linker (>10 atoms) attached to the support matrix since it can avoid the steric hindrance (The ligand is inaccessible to the binding site). Linker length is less critical for large (e.g., protein) antigens since the antigen itself is an effective linker between the support matrix and the epitope.
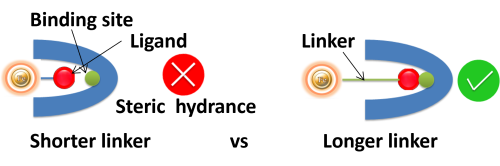
●
Site-directed immobilization: Using a unique functional group for site-directed immobilization is beneficial for correct orientation such as sulfhydryl on a single terminal cysteine in a peptide for a small antigen and carbohydrate residues on the Fc side for antibody.
●
Use hydrophilic linker to immobilize antigen to activated support to reduce background noise.
●
Use a cleavable linker to separate the target from the solid matrix easily.
Immobilization chemistries
Affinity ligands can be linked (immobilized) to the solid substance (stationary phase) in a variety of ways.
The copper-catalyzed azide-alkyne cycloaddition (CuAAC) is an efficient and reliable reaction that uses the copper-catalyzed coupling of azides to terminal acetylenes via powerful linking (1, 4-disubstituted triazole) to produce unique practical and versatile new biological compounds. CuAAC, as the most used click chemistry, is widely used in bioconjugation, drug discovery, materials science, and radiochemistry due to its numerous unique advantages.
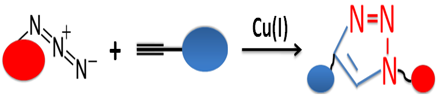
Feature and Benefits
1.
Fast kinetics and high efficiency
The tetrazine cycloaddition reaction is extremely fastand takes only minutes to complete. Due to such a highreaction rate, it works so well even with an ultra-low concentrationof reagents for most biological systems. Moreover, the reaction produces minimal byproducts but quantitative yields. Rapid and quantitative labeling allows non-radioactive analysis of enzymatic activities both in vitro and in vivo: Small-sized CLICK-functional groups possess excellent substrate properties.
2.
Outstanding selectivity and specificity
The azide or alkynes moiety is absent in almost all native biomolecules, and the reaction occurs only between terminal alkynes and azides groups. It is performed in the presence of other functional moieties, which allow selective ligation with a very limited set of reaction partners.
3.
Very flexibility
The small size and remarkable stability of azide, alkyne, or linkage (1, 4-disubstituted triazole) are proven to be exceptionally suitable for relatively easy incorporation into a variety of molecules such as synthetic polymers, fluorophores, small molecules, or into specific locations in biomolecules, that imposes minimal perturbation to the structure and function of the conjugated biomolecules.
4.
Mild reaction condition
The reaction can be performed under various reaction conditions, including various solvents such as organic solvent, pure water, buffers, a wide pH (pH 4-11), and temperature range.
Applications
To meet the increasingly demanding applications in using azide-alkyne click reactions, we provide Alkyne and Azide-activated magnetic beads-based systems as more efficient tools.
Alkyne and Azide-activated magnetic beads
The activated magnetic beads are uniform inert silica-based magnetic beads grafted with a high density of alkyne or azide functional groups on the surface. The beads are designed to efficiently enrich alkyne or azide-tagged biomolecules from complex cell lysates via a Cu(I)-catalyzed Alkyne-Azide (CUAAC) reaction. Compared with other affinity resins such as agarose beads or other polymers, the inert silica enclosed magnetic beads offer high stability, low nonspecific binding, and superior handling in protein-based systems. Since the active cleavable alkyne or azide group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads after affinity purification. These magnetic beads are ideal tools for genomics, proteomics, biomarker discovery, posttranslational modification (PTM) analysis, etc.
Learn More
The amine group (-NH2) is the most common functional target for immobilizing protein molecules: This group can be found at the N-terminus of each polypeptide chain (called the alpha-amine) and in the side chain of lysine (Lys, K) residues (called the epsilon-amine). Because of their positive charge under physiological conditions, primary amines are frequently outward-facing (i.e., on the outside surface) of proteins; hence, they are usually available for conjugation without denaturing the protein structure.
BcMag™ NHS-Activated Magnetic Beads are uniform, magnetic beads coated with high-density NHS (N-hydroxyl succinimide) functional groups on the surface. The magnetic resin utilizes reliable NHS-ester chemistry and does not require the use of dangerous chemicals for immobilization. The beads have less nonspecific binding and no leaking of the coupled ligand. The resin can quickly, efficiently, and covalently conjugate any primary amine-containing ligands by forming stable amide linkages with better than 85% coupling efficiency. Bioclone NHS-Activated beads reactions take less than an hour (15–30 min at room temp pH 7–9, 4 hours at 4 °C) and produce substantially more stable linkages. BcMag™ Long-arm NHS-activated Magnetic Beads are recommended to immobilize small peptides because the long-arm (21-atom) hydrophilic linker may reduce steric hindrance. The magnetic resin can be employed in various affinity purification methods.
BcMag™ Cleavable NHS-Activated Magnetic Beads are uniform magnetic beads coated with high density of cleavable NHS (N-hydroxyl succinimide) functional groups on the surface. The beads can easily, efficiently and covalently conjugate any primary Amine-containing ligands through forming stable amide linkages. Since the active NHS group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
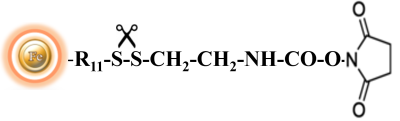
The unique dry form eliminates the need for acetone solvent storage or removal and disposal. Furthermore, because the dry resin concentrates the sample as it swells, lowering the volume of the starting material and resulting in highly effective ligand immobilization, it is perfect for coupling reactions with dilute materials.
The NHS magnetic resins are used to replace time-consuming, complex, and costly chromatographic procedures such as agarose, cellulose, sepharose, and sephadex-based columns or resins. In column-based processes, the lysate is centrifuged or cleared, the supernatant is added to the column, the membrane or resin is washed with buffer through centrifugation or vacuum manifold, and the required biomolecules are eluted in an adequate volume of buffer. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in target biomolecule yield between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields.
Features and Advantages
●
Pre-activated and ready-to-use
●
Easy to use
●
A quick coupling in pH 7.4 at 4°C to 25°C
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable immunoaffinity matrices
●
Low nonspecific binding
●
Immobilize 1-10 mg protein or 0.1-1 mg peptide/ml beads
●
Applications: Cell sorting, Immunoprecipitation; Purification for Antibodies, Proteins/Peptides, DNA/RNA
Learn More
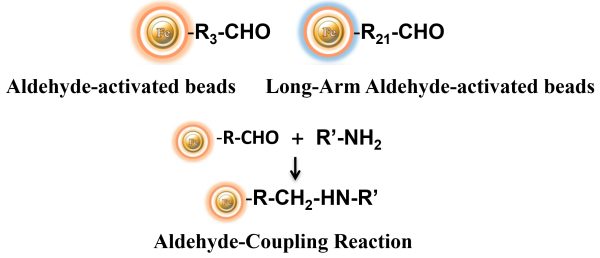
The Aldehyde-Activated resins have higher coupling efficiency than cyanogen bromide (CNBr) activated supports. Furthermore, the aldehyde Chemistry generates an uncharged connection with the amine-containing ligand that is more stable than the CNBr approach. The hydrophilic surface ensures excellent dispersion and easy handling in various buffers. When utilized for affinity purification methods, these features allow better leak-resistant immobilization and lower nonspecific binding. BcMag™ Aldehyde-Activated Magnetic Beads are suitable for conjugating a large protein. At the same time, BcMag™ Long-arm Aldehyde-Activated Magnetic Beads are ideal for conjugating small peptides because the long-arm hydrophilic linker may reduce steric hindrance.
BcMag™ Cleavable Aldehyde-Activated Magnetic Beads are uniform, silica-based superparamagnetic beads grafted with a high density of aldehyde functional groups on the surface. In a one-step process, the beads can efficiently covalently conjugate primary amine-containing ligands such as proteins and other biomolecules. Since the active aldehyde group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads, and only a small sulfhydryl group is attached to ligand after affinity purification. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.

Features and Benefits
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Stable covalent bond with minimal ligand leakage
●
High capacity – Immobilize 15 -20 µg antibody/mg beads
●
Scalable – easily adjusts for sample size and automation
●
Low nonspecific binding
●
Reproducible results
●
Application: Purification for antibody, protein/peptide, DNA/RNA, cell sorting, immunoprecipitation
Learn More
CDI-activated support is another alternative for immobilizing amine-containing affinity ligands. When the support reacts with primary amine-containing ligands in an aqueous coupling buffer, the imidazole groups are lost and carbamate linkages are formed. The coupling process occurs at basic pH (8.5-10), however with proteins it is a slower reaction than reductive amination coupling. Peptides and tiny chemical compounds are particularly well-immobilized by CDI-activated resins. The reaction can also be carried out in organic solvent to allow the coupling of water-insoluble ligands.
BcMag™ CDI-Activated Magnetic Beads is a carbonyldiimidazole affinity chromatography resin that has been activated for covalent immobilization of N-nucleophiles and primary amine ligands at pH 9 to 11 in aqueous or organic solvent conditions and are particularly suitable for conjugating water-insoluble peptides and small organic molecules in organic solvent. BcMag™ CDI-activated magnetic beads are ideal for conjugating a large protein or small peptide. The unique dry form eliminates the need for acetone solvent storage or removal and disposal.
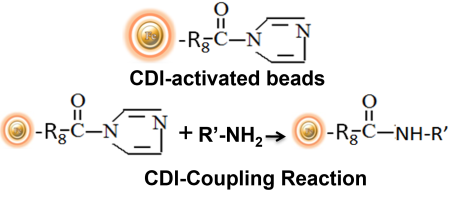
Features and Benefits
●
Reliable coupling chemistry – immobilization occurs as a result of the reaction of N-nucleophiles with the resin’s 1,1′-carbonyldiimidazole groups, which results in the formation of stable, uncharged N-alkylcarbamate linkages.
●
Simple to use – no secondary reagents are required; simply wash and equilibrate the resin in alkaline coupling buffer before adding the ligand; the reaction proceeds spontaneously
●
Stable activation – the half-life of hydrolysis is much longer than that of hydroxysuccinimide ester activations, making immobilization reactions easier to prepare and control.
Learn More
It is frequently useful to immobilize affinity ligands via functional groups other than amines. The thiol group, in particular, can be employed to guide coupling processes away from active centers or binding sites on specific protein molecules. Sulfhydryls, commonly known as thiols, are found in proteins as side chains of cysteine (Cys, C) amino acids. As the foundation of native tertiary or quaternary protein structure, pairs of cysteine sulfhydryl groups are frequently linked via disulfide bonds (-S-S-) within or between polypeptide chains. For most thiol-reactive compounds, only free or reduced sulfhydryl groups (-SH) [rather than sulfur atoms in disulfide bonds] are available for reaction.
Advantages of protein conjugation via sulfhydryl groups:
First, sulfhydryls are found in most proteins but are not as abundant as primary amines, immobilizing via sulfhydryl groups more selective and precise. Second, because sulfhydryl groups in proteins are frequently engaged in disulfide bonds, crosslinking at these locations usually has little effect on the underlying protein structure or blocks binding sites. Third, the quantity of accessible (i.e., free) sulfhydryl groups can be easily regulated or manipulated; they can be formed by reducing native disulfide bonds or introduced into molecules via reactivity with primary amines. Finally, combining sulfhydryl-reactive groups with amine-reactive groups to form heterobifunctional crosslinkers allows for more flexibility and control over crosslinking methods.
Iodoacetyl-activated Magnetic Beads
Supports with iodoacetyl immobilization
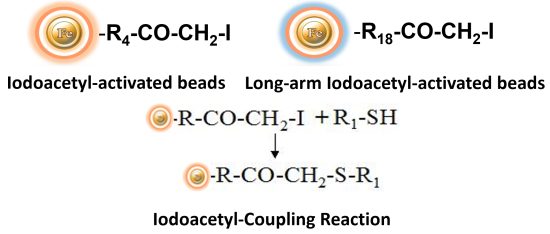
Protein immobilization to magnetic beads or beaded agarose resin is one application where haloacetyls remain the more preferred sulfhydryl-reactive chemical. BcMag™ Iodoacetyl-activated beads and kits can be used to immobilize half-antibodies (as described above) or protein subunits with reduced cysteine disulfide linkages. However, the most important and widely utilized application of iodoacetyl-activated bead is to immobilize cysteine-containing peptides for antibody purification after immunization and antibody generation with the same peptide.
BcMag™ Iodoacetyl-activated Magnetic Beads are uniform, silica-based superparamagnetic beads coated with a high density of Iodoacetyl functional groups on the surface. It is designed to enable fast, efficient, and covalent immobilization of protein, peptides, and other ligands through their sulfhydryl groups (-SH) for affinity purification procedures. At physiological to alkaline circumstances (pH 7.2 to 9) in either aqueous or organic solvents 20- 30% DMSO or DMF, iodoacetyl-activated supports react with sulfhydryl groups, resulting in stable thioether bonds. These reactions are often carried out in the dark to prevent the formation of free iodine, which has the ability to react with tyrosine, histidine, and tryptophan residues. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. BcMag™ Iodoacetyl-activated Magnetic Beads are most suitable for conjugation of a larger protein. BcMagTM Long-arm Iodoacetyl-activated Magnetic Beads are recommended for conjugation of small peptides because the long-arm (20-atom) hydrophilic linker may reduce steric hindrance.
BcMag™ Cleavable Iodoacetyl-activated Magnetic Beads are uniform, silica-based superparamagnetic beads coated with high density of cleavable iodoacetyl functional groups on the surface. It is designed to enable fast, efficient and covalent immobilization of protein, peptides and other ligands through their sulfhydryl groups (-SH) for use in affinity purification procedures. Since the active a iodoacetyl group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads, and only a small sulfhydryl group is attached to ligand after affinity purification. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The beads are suitable for conjugation of larger protein or small biomoleculs without steric hindrance problem.
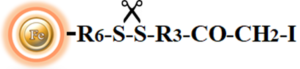
Features and Benefits
●
Iodoacetyl groups react selectively with sulhydryl (-SH) groups to produce irreversible thioether linkages.
●
Fast – couple peptide samples in 2 hours.
●
Cleavable built-in disulfide bond allows the ligand-target molecule complex separated from the beads.
●
Versatile coupling conditions – as needed for protein or peptide solubility during the coupling reaction, employ pH 7.5 to 9.0 aqueous buffers, organic solvent (e.g., 20% DMSO), or denaturant (guanidineHCl).
●
Simple to follow protocols for sample preparation, immobilization, and affinity purification
●
High capacity – Immobilize 15 -20 µg antibody/mg beads.
Applications
●
Immobilize peptides with terminal cysteine residues to purify antibodies raised against peptide immunogens.
●
Immobilize antibodies in an orientated manner using hinge-region sulfhydryls to ensure that antigen binding sites are not sterically inhibited when antigen affinity purification is performed.
●
Produces reusable immunoaffinity matrices
●
Maintains antibody function – immobilizes IgG via the Fc region, leaving both antigen binding sites available for target capture.
Learn More
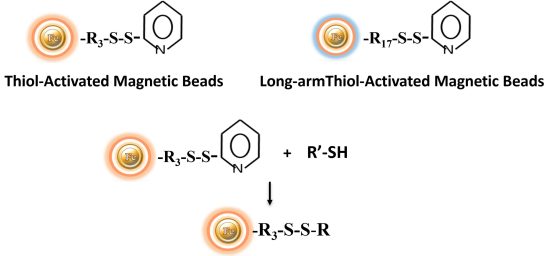
Chemistry of the pyridyl disulfide reaction: Pyridyl disulfides form disulfide connections with sulfhydryl groups over a wide pH range (the optimum is pH 4 to 5). A disulfide exchange happens during the reaction between the molecule’s -SH group and the reagent’s 2-pyridyldithiol group. These chemicals can be used to crosslink proteins and introduce sulfhydryl groups. The disulfide exchange can be conducted at physiological pH, however the reaction rate is slower.
Applications for pyridyl disulfide immobilization
Because pyridyldithiol compounds generate disulfide bonds with target sulfhydryls, conjugates created with these crosslinkers are cleavable using common disulfide reducing agents, such as dithiothreitol (DTT) or sample buffer for protein electrophoresis (SDS-PAGE). Thus, pyridyldisulfide magnetic beads are useful alternatives to maleimide and haloacetyl reagents when it is necessary to reverse the sulfhydryl-conjugation step later in an experimental procedure (i.e., to exactly recover the original sulfhydryl-containing molecule).
BcMagTM Thiol-activated Magnetic Beads are uniform magnetic beads coated with high-density thiol functional groups (2-pyridyl disulfide) on the surface. The beads can reversibly immobilize thiol-containing ligands under mild conditions. After affinity purification, reducing agents such as DTT or β-mercaptoethano can cleave and separate the target molecule-ligand complex from the beads. BcMag™ thiol-activated magnetic beads are most suitable for conjugation of large proteins. BcMag™ long-arm thiol-activated Magnetic Beads are recommended for conjugation of small peptides because the long-arm hydrophilic linker may reduce steric hindrance.
Features and Advantages
●
Pre-activated and ready-to-use
●
Cleavable built-in disulfide bond allows the ligand-target molecule Complex separated from the beads.
●
Specific isolation of cysteine proteins/peptides
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable affinity matrix
●
Low nonspecific binding
●
Applications: Affinity purification, immunoprecipitation, purification of antibodies, proteins/peptides, DNA/RNA
Learn More
In their natural condition, most biological compounds do not contain carbonyl ketones or aldehydes. However, similar groups on proteins may be effective for forming an immobilization site that drives covalent coupling away from active centers or binding regions. Glycoconjugates, such as glycoproteins and glycolipids, typically contain sugar residues with hydroxyls on neighboring carbon atoms; these cis-diols can be oxidized with sodium periodate to produce aldehydes as covalent immobilization sites.
BcMag™ Hydrazide-Terminated Magnetic Beads are uniform magnetic beads grafted with a high density of hydrazide functional groups on the surface. Hydrazide chemistry is effective for labeling, immobilizing, or conjugating glycoproteins via glycosylation sites, which are frequently (as with most polyclonal antibodies) positioned in domains distant from the critical binding sites whose function needs to be preserved. Coupling antibodies in this manner selectively targets heavy chains in the Fc portion of the molecule, assisting in the best possible preservation of antigen binding activity by the ends of the Fv regions. At pH 5 to 7, hydrazide-terminated supports and compounds will conjugate with carbonyls of oxidized carbohydrates (sugars), resulting in the production of hydrazone linkages. The hydrazide beads are suitable for conjugating larger glycoproteins and glycolipids, carbohydrates or other ligands. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
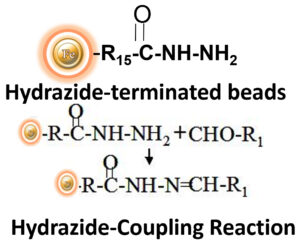
BcMag™ Cleavable Hydrazide-Terminated Magnetic Beads are uniform magnetic beads grafted with a high density of hydrazide functional groups on the surface. The hydrazide beads are suitable for conjugating larger glycoproteins and glycolipids, carbohydrates or other ligands. Since the active hydrazide group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads after affinity purification. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.

BcMag™ Glycoprotein & Antibody Conjugation Kit produces affinity support with glycoproteins and antibodies, which often have numerous carbohydrates (glycosylation) on their Fc sections, using hydrazide-activated magnetic beads and a specific catalyst. These kits may immobilize any glycosylated proteins (including monoclonal antibodies) with periodate-oxidizable carbohydrate groups to create reusable affinity matrices. This hydrazide immobilization chemistry results in antibodies with unobstructed antigen-binding sites and excellent purification capacity. In a simple non-amine buffer containing the catalyst, the full coupling reaction takes 2 to 4 hours. Coupling efficiency with antibodies and common glycoproteins are typically greater than 85%, yielding 15µg to 20 µg of immobilized protein per mg of the hydrazide-terminated magnetic beads. The matrix prepared with stable glycoproteins or antibodies can be regenerated and reused at least five times without appreciable loss of binding capacity.
Features and Benefits
●
Covalently coupling with cleavable built-in disulfide bond allows the ligand-target molecule complex separated from the beads.
●
Specific immobilization – the hydrazide-activated beads bind exclusively to pure glycoproteins containing sugar groups that have been gently oxidized with periodate (e.g., sialic acid).
●
Stable covalent bond with low levels of ligand leakage
●
Maintains antibody function – immobilizes IgG via the Fc region, leaving both antigen binding sites available for target capture.
●
Low nonspecific binding
●
High capacity – Immobilize 15-20µg antibody/mg beads
Learn More
The carboxyl group is found in a wide range of biological substances. Peptides and proteins contain carboxyls (-COOH) at the C-terminus of each polypeptide chain as well as aspartic acid (Asp, D) and glutamic acid side chains (Glu, E). Carboxyls, like primary amines, are typically found on the surface of protein structures.
Through the application of a carbodiimide-mediated process, carboxylic acids can be employed to immobilize biological molecules. Despite the fact that no activated support has a reactive group that is spontaneously reactive with carboxylates, chromatographic supports containing amines (or hydrazides) can be utilized to create amide bonds with carboxylates activated using the water-soluble carbodiimide crosslinker EDC.
BcMag™ Amine-terminated Magnetic Beads are magnetic beads coated with a high density of primary amine functional groups on the surface. The beads can covalently conjugate primary amine or carboxy-containing ligands such as protein, peptides for affinity purification. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. BcMag™ Amine-Terminated Magnetic Beads are most suitable for the conjugation of a larger protein. BcMagTM Long-arm amine-terminated Magnetic Beads are recommended for conjugation of small peptides because the long-arm (21-atom) hydrophilic linker may reduce steric hindrance.
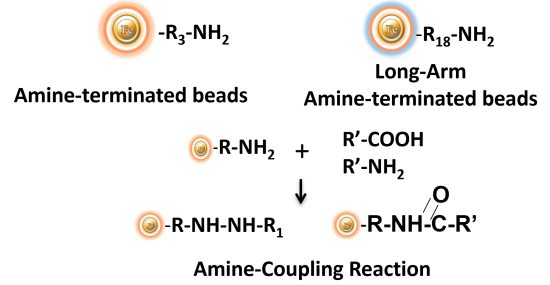
BcMag™ Cleavable Amine- Terminated Magnetic Beads are magnetic beads coated with a high density of primary amine functional groups on the surface. The beads can covalently conjugate primary amine or carboxy-containing ligands such as protein, peptides for affinity purification. Since the active amine group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The beads are suitable for conjugation of large-size protein or small peptide.
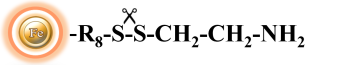
Features and Benefits
●
Covalently coupled with high efficiency
●
Easy to use
●
Stable covalent bond with low levels of ligand leakage
●
Produces reusable immunoaffinity matrix
●
Low nonspecific binding
●
Immobilize 15-20 µg antibody/mg beads
●
Cleavable built-in disulfide bond allowing the ligand-target molecule complex separated from the beads.
●
Application: Purification for antibody, protein/peptide, DNA/RNA, cell sorting, immunoprecipitation
Learn More
BcMag™ Carboxyl-Terminated Magnetic Beads are uniform, silica-based magnetic beads coated with a high density of carboxylic acid groups on the surface. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The high density of pendent functional carboxylic acid groups on their surface allows covalently conjugate primary amine-containing ligands via a stable amide bond. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. BcMag™ Carboxyl-Terminated Magnetic Beads are most suitable for conjugating a larger protein. At the same time, BcMagTM Long-arm Carboxyl-terminated Magnetic Beads are recommended for conjugation of small peptides because the long-arm hydrophilic linker may reduce steric hindrance.
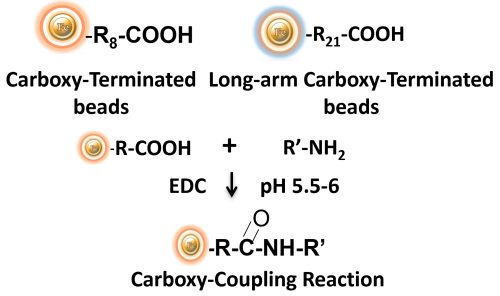
BcMag™ Cleavable Carboxyl-Terminated Magnetic Beads are uniform, silica-based magnetic beads coated with a high density of carboxylic acid groups on the surface. The high density of carboxylic acid groups on their surface allows efficiently covalently conjugate primary amine-containing ligands via a stable amide bond. Since the active carboxyl group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads. The cleavable carboxyl magnetic beads are ideal matrices for conjugating large size protein or small peptide. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.

EDC and other carbodiimides are zero-length crosslinkers that cause carboxylates (-COOH) to conjugate directly to primary amines (-NH2) without becoming part of the ultimate crosslink (amide bond) between target molecules. When diaminodipropylamine (DADPA) agarose resin is employed as the primary amine in this process, ligand immobilization occurs.
Because peptides and proteins contain numerous carboxyls and amines, direct EDC-mediated crosslinking typically results in polypeptide random polymerization. However, this reaction chemistry is commonly utilized in immobilization methods (for example, binding proteins to a carboxylated surface) and immunogen production (e.g., attaching a small peptide to a large carrier protein). Indeed, some peptide polymerization is advantageous in antibody manufacturing and purification operations because it boosts overall peptide conjugation (loading) and ensures that the peptide ligands are supplied in numerous orientations.
Learn More
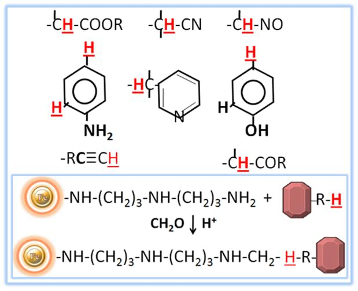
Immobilization using traditional procedures may be difficult, if not impossible, for molecules lacking easily reactive functional groups. Certain medications, steroids, dyes, and other small chemical molecules, in particular, commonly have structures that lack appropriate reaction groups for immobilization. Other compounds have low-reactivity functional groups or are sterically inhibited. However, some of these compounds contain active (or replaceable) hydrogens that can be condensed using the Mannich method with formaldehyde and an amine.
Mannich reaction-induced immobilization
The Mannich reaction is defined as the condensation of formaldehyde (or another aldehyde) with ammonia and another molecule containing active hydrogen. Instead of ammonia, this reaction can be carried out with primary, secondary, or even amide amines. When diaminodipropylamine (DADPA) resin is used as the primary amine in this process, ligand immobilization occurs.
The BcMag™ Steroid/Drug/Dye Conjugation Kit use DADPA-Terminated Magnetic Beads to efficiently conjugate compounds that lack easily reactive functional groups, such as steroids, dyes, and medications, via Mannich reaction-induced immobilization. BcMag™ DADPA-Terminated Magnetic Beads are uniform, silica-based superparamagnetic beads coated with high density of DADPA (Diaminodipropylamine) functional groups on the surface. Steroids, dyes, drugs and etc are classes of small molecules, which are difficult or impossible to be immobilized by current immobilization methods due to either absence or inaccessibility of easily reactive functional groups. However, those small molecules may be possibly immobilized to DADPA-terminated magnetic beads via their active hydrogens being condensed with formaldehyde and an amine in the Mannich reaction.
Features and Benefits
●
High binding capacity
●
Fast, efficient coupling
●
Hydrophillic long-arm spacer minimizing steric hindrance and non-specific binding.
Learn More
BcMag™ Epoxy-Activated Magnetic Beads are pre-activated, uniform magnetic beads coated with high-density epoxy functional groups on the surface. The beads can covalently conjugate amine, sulfhydryl, or hydroxyl group-containing ligands. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The epoxy-activated magnetic beads are most suitable for the conjugation of large proteins. BcMag™ Long-arm Epoxy-Activated Magnetic Beads are recommended for conjugation of small peptides because the long-arm hydrophilic linker may reduce steric hindrance.
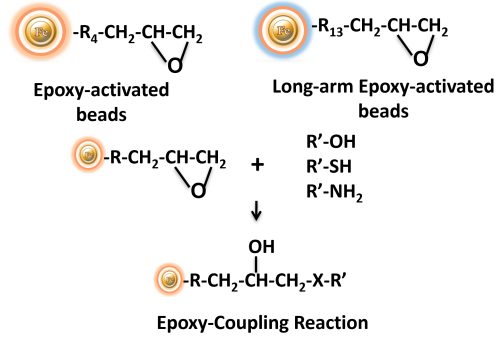
BcMag™ Cleavable Epoxy-Activated Magnetic Beads are pre-activated, uniform magnetic beads coated with high-density epoxy functional groups on the surface. The beads can covalently conjugate amine, sulfhydryl, or hydroxyl group-containing ligands. Since the active epoxy group is linked with the beads through a built-in cleavable disulfide linker, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The beads are suitable for the conjugation of large proteins or small peptides.

Any ligands such as antibodies, peptides, complete proteins, and functional enzymes can be covalently linked to the surface of the bead in a simple overnight reaction. Simply incubate the ligand you intend to use for cell isolation with these epoxy activated beads at high pH (8.5-9.5) and 37°C. Coupling can be done in an alternate buffer at pH 7.4 for pH labile ligands.
The unique dry form eliminates the need for acetone solvent storage or removal and disposal. Furthermore, because the dry resin concentrates the sample as it swells, lowering the volume of the starting material and resulting in highly effective ligand immobilization, it is perfect for coupling reactions with dilute materials
Features and Advantages
●
Pre-activated and ready-to-use
●
Cleavable built-in disulfide bond allows the ligand-target molecule complex separated from the beads.
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable immunoaffinity matrices
●
Low nonspecific binding
●
Immobilize 1-10 mg protein or 0.1-1 mg peptide/ml beads
●
Applications: Immunoprecipitation, purification for Antibodies, Proteins/Peptides, DNA/RNA
Learn More
BcMagTM Tosyl Activated Magnetic Beads are magnetic beads grafted with tosyl functional groups on the surface. Short and long-arm tosyl activated magnetic beads can efficiently conjugate ligands containing primary amine in either aqueous or organic solvents (30% DMF) without introducing any charge. The Tosyl Activated Magnetic Beads are suitable for conjugation of large size protein, while long-arm tosyl activated magnetic beads are ideal for coupling small molecules without steric hindrance problem.
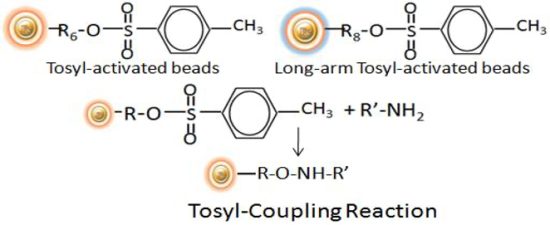
BcMagTM Cleavable Tosyl-Activated Magnetic Beads are uniform magnetic beads grafted with tosyl functional groups on the surface.). Since the active tosyl group is linked with the beads through a built-in cleavable disulfide linker, after affinity purification, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads. The cleavable tosyl magnetic beads are ideal matrices for conjugating large-size proteins or small peptides.
The Tosyl Activated magnetic resins are ideal choice for covalently attaching antibodies, peptides, complete proteins, and functional enzymes to the surface. The immobilized beads are widely used in Immunoprecipitation of proteins and protein complexes due to their low background and covalent binding of antibodies to the bead surface.
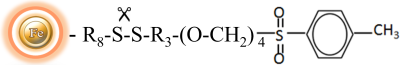
The Tosyl Activated magnetic resins coupling reaction is carried out at 37°C and pH ranges from neutral to high. We advocate coupling at pH 8.5-9.5, but coupling with pH labile ligands can be done in an alternate buffer at pH 7.4.
The unique dry form eliminates the need for acetone solvent storage or removal and disposal. Furthermore, because the dry resin concentrates the sample as it swells, lowering the volume of the starting material and resulting in highly effective ligand immobilization, it is perfect for coupling reactions with dilute materials.
Learn More
BcMag™ DVS-Activated Magnetic Beads are pre-activated and uniform beads coated with high-density DVS (Divinyl Sulfone) functional groups on the surface. The beads can covalently conjugate primary amino, sulfhydryl, or hydroxyl groups-containing ligands. The matrix is unique affinity support for immobilizing sugars and carbohydrates through the hydroxyl groups present on carbohydrates. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. BcMag™ DVS-activated magnetic beads are an ideal affinity matrix for immobilizing large molecules or small peptides because the long-arm hydrophilic linker may reduce steric hindrance.
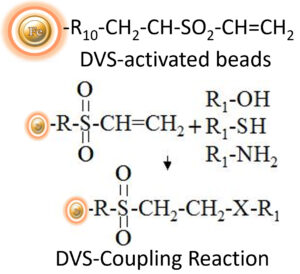
Features and Advantages
●
Pre-activated and ready-to-use
●
Easy to use
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable immunoaffinity matrices
●
Low nonspecific binding
●
Immobilize 1-10 mg protein or 0.1-1 mg peptide/ml beads
●
Applications: Immunoprecipitation, Purificationfor Antibodies, Proteins/Peptides, DNA/RNA
Learn More
Magnetic Beads Make Things Simple