- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

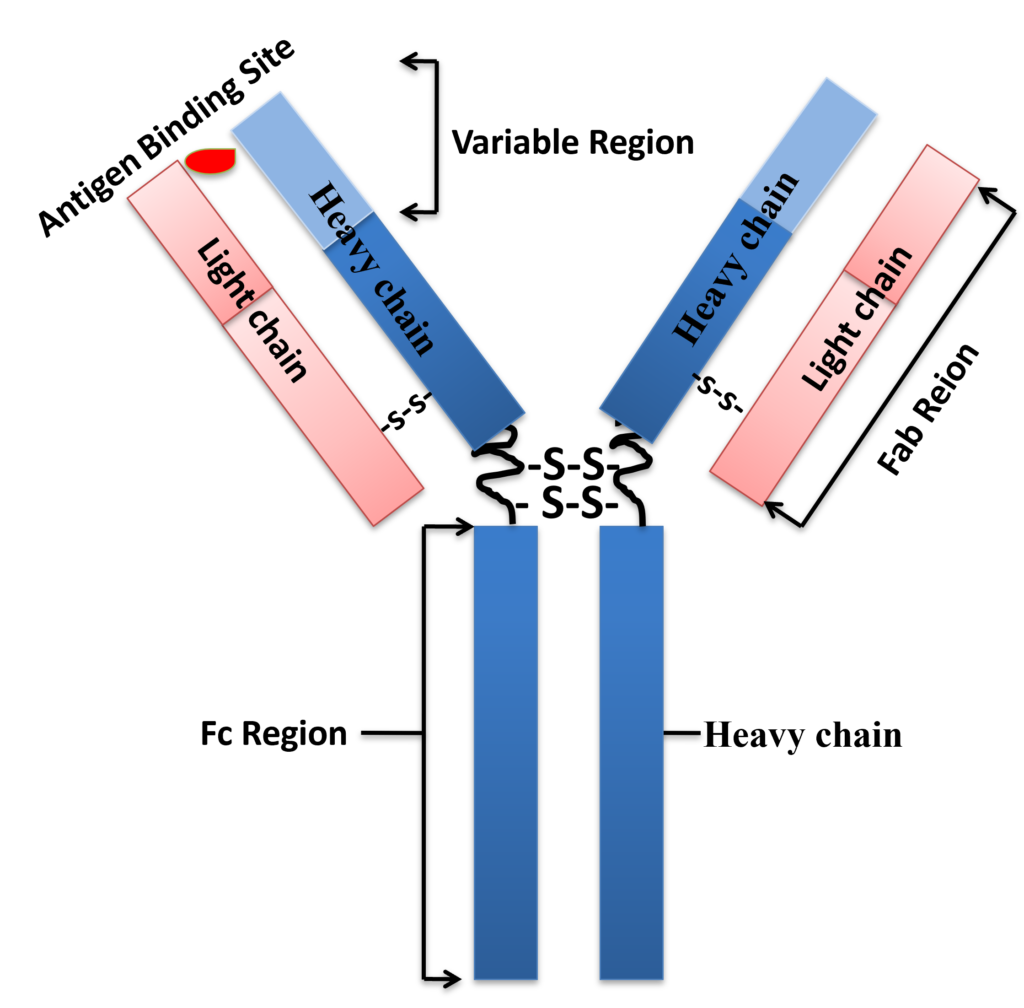
Antibodies (also known as immunoglobulin) are Y-shaped large glycoproteins produced by the immune system to identify and defend cells from foreign invading pathogenic bacteria or viruses. The Y-shaped immunoglobulin consists of two identical heavy chains and two identical light chains, which are linked together by disulfide bonds. The two heavy chains are held together by disulfide bonds, and each heavy chain is connected to a light chain by a disulfide bond, forming a symmetrical “Y” molecule. The variable domains of heavy and light chains from the antigen-binding site (Fig.1). These antibodies are categorized into two main types: monoclonal and polyclonal. Polyclonal antibodies contain a heterologous mixture of IgGs produced by different B cells and recognize different epitopes (The small site on an antigen to which a complementary antibody may specifically bind) on the same antigen. Monoclonal antibodies are a homogenous antibody population produced from a single B-cell and recognize and bind to only one epitope on a single antigen. In mammals, antibodies are classified into five isotypes (IgG, IgM, IgA, IgD, and IgE) based on immunoglobulin structure.
●
IgG is a monomer (single Ig uint) and the main antibody, making up 75-85% of the total immunoglobulin in blood and other body fluids. It has four subclasses (IgG1, IgG2, IgG3, and IgG4) that provide immunity against foreign pathogens and can also cross the placenta and provide fetal immunity.
●
IgM: IgM is produced as a monomer when expressed on the surface of B cells or as five or rarely of six subunits (Ig unit) when it is secreted. IgM antibodies function as a body’s first protection response to a new infection, serving short-term protection. They increase for a couple of weeks and then decrease as IgG production begins.
●
IgA: IgA is a monomer in the blood formed as dimmers (two Ig units) in secretions such as bowel fluid, nasal discharge, saliva tears, and breast milk. IgA constitutes about 15% of the total antibodies in the blood. It t provides the first line of defense against invading bacterial and viral pathogens.
●
IgD: IgD is produced in a secreted form and serves as an antigen receptor, which binds allergens, subsequently triggering histamine release from mast cells. It consists of 0.25% of immunoglobulins in serum.
●
IgE is produced by immune reactions to parasites and allergies such as pollinosis.
●
In contrast to mammalian species, IgY is the most common immunoglobulin in birds, reptiles, amphibians, and lungfish. IgY has a structure quite similar to IgG but has a different constant domain within the IgY heavy chain.
Because of their high specificity and selectivity, antibodies have become a powerful biochemical tool for modern medicine, laboratory science, and environmental monitoring. Monoclonal antibodies (mAbs), polyclonal antibodies (polyclonal antibodies), recombinant antibodies, and antibody fragments have different applications that demand different purification procedures. Their applications include immunodiagnostics, immunotherapeutics, drug targeting, detection of foodborne pathogens, contaminants, toxins, and residues in food samples, environmental analysis/monitoring, and isolation of a variety of proteins and other targets for basic research and industrial processes, including but not limited to: Flow Cytometry, Immunoassays, and ELISA, Immunofluorescence, Immunohistology / Immunohistochemistry, Immunoprecipitation, Western Blotting.
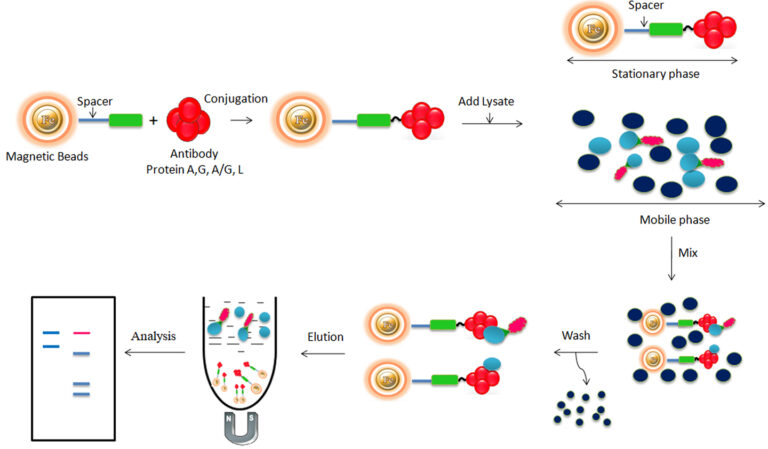
Antibodies can be separated from complicated mixtures using either chromatographic or non-chromatographic approaches. Separating antibodies or antibody-derived molecules from complex mixtures using chromatography entails passing them through a solid phase (e.g., silica resin or beads, monolithic columns, or cellulose membranes) and allowing the antibodies to bind or pass through depending on whether “bind-and-elute” or “flow-through” chromatographic methods are used. In chromatographic procedures, separation techniques such as affinity-tag Binding, ion-exchange, size-exclusion chromatography, and immunoaffinity chromatography can all be used.
MAbs are isolated from mammalian cells such as Chinese hamster ovary (CHO) cells in batches. MAbs are commonly utilized as therapies in treating cancer, autoimmune disorders, and inflammatory diseases; these entities require the highest level of purification before they are appropriate for patient administration. Impurities such as endogenous viruses (used during antibody creation), DNA, host cell proteins, endotoxins, and aggregates must be removed since they can activate the patient’s immune system. Removal can be accomplished using various methods, the most common combinatorial procedures such as using antibody-binding protein affinity chromatography followed by anion-exchange chromatography and antigen-specific antibody chromatography purification.
Protein A, G, A/G, and L are native and recombinant proteins whose antibody-binding properties have been well characterized. The native proteins are replaced by the recombinant proteins produced in E.coli since the recombinant proteins have higher capacity, are highly robust, and have maximum specific antibody binding.
Protein A, Protein G, or Protein A/G binds with high affinity to the Fc portion of various classes and subclasses of immunoglobulins from various species by independent and separate binding sites. Protein L binds the different antibody isotypes (IgG, IgM, IgA, IgE, and IgD) through the interaction with the variable domain of the Ig kappa light chain with no interference with an antibody’s antigen-binding site. These proteins vary in their ability to bind to different subtypes and species antibodies. Therefore, choosing the antibody-binding proteins to match the corresponding antibody is essential for your application.
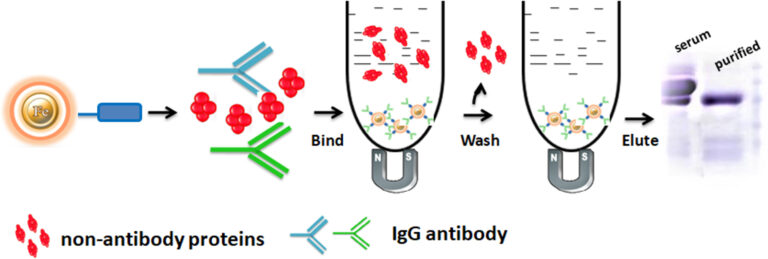
Bioclone develops a great antibody affinity chromatography matrices – Protein A, G, A/G, and L magnetic beads – used for antibody purification from serum, cell culture supernatant, or ascites, as well as antigen IP/Co-IP from cell or tissue extracts. The procedure for those Magnetic Beads has been improved to allow maximum recovery and purity of the recovered antibody or antigen. For antibody purification, the beads are incubated with the antibody solution, which is magnetically separated from the supernatant. For immunoprecipitation, the beads are delivered to an antigen-containing sample to which an antibody has been introduced and allowed to incubate to form the antibody-antigen complex. The attached antibodies or antigens are dissociated from the beads using an elution buffer and recovered from the solution manually using a magnetic rack or by using automation instruments.
Workflow
1.
Bind: Add a clarified, physiologic-buffered (pH 7 to 8) serum sample to the beads and allow the antibody to mix and bind to the immobilized ligand.
2.
Wash: Wash the beads to remove the nonspecific binding protein.
3.
Elute: Elute antibody from the beads by an acidic elution buffer (e.g., 0.1 M glycine-HCl, pH 2.8).
4.
Neutralize the antibody: Add 1/10th volume of 1 M Tris-HCl (pH 8.5) to neutralize the buffer.
Explore Products
Polyclonal antibodies (serum samples) are a mixture of unrelated IgG and antigen-specific IgG. The antigen-specific IgG generally accounts for only 2-5% of total IgG in serum. The antigen-specific IgG is often required to quantify the relative amount of the antigen-specific antibody and for applications such as ELISA, neutralization, immunohistochemistry, and Western blotting since large quantities of the unrelated antibody can increase background noise. Proteins A, G, A/G, or L are excellent tools for purifying total IgG from various samples, but with the problem of co-purification of nonspecific immunoglobulins. Therefore, it cannot purify antigen-specific antibodies from the antiserum. Isolation of these antibodies by other purification methods (e.g., ion-exchange chromatography) is tedious and ineffective. On the other hand, immunoaffinity purification, which exploits the specificity of antibody-antigen interactions, provides many advantages such as very high purity levels (>95% can be achieved by using a specific 3-step procedure), very high selectivity, and therefore very high resolution. Antigen-specific affinity purification of antibodies involves antigen immobilization to a solid support and antibody purification from the serum.
Any molecule that induces the immune system to develop antibodies against it is referred to as an antigen. Antigens are typically proteins or polysaccharides with a large molecular weight. Antigens can also be polypeptides, lipids, nucleic acids, and various other substances. Immune responses can be elicited against smaller molecules known as haptens when they are chemically attached to a bigger carrier protein such as bovine serum albumin, keyhole limpet hemocyanin (KLH), or other synthetic matrices. Haptens can be compounds such as medicines, simple sugars, amino acids, short peptides, phospholipids, or triglycerides. As a result, given enough time, almost any foreign material will be recognized by the immune system and elicit particular antibody formation. However, this unique immune response is highly varied and is heavily influenced by antigen size, shape, and composition. Proteins or glycoproteins are thought to be the best antigens because of their potential to elicit a potent immune response; in other words, they are highly immunogenic.
Making antigen-specific affinity supports requires methods to covalently conjugate antigens to an appropriate insoluble support (matrix). Antigen-specific affinity purification of antibodies involves two processes: antigen immobilization to a solid support and antibody purification procedure from the serum. Immobilization and conjugation refer to the covalent attachment of an antigen to a solid support through their common chemical groups. In this method, the matrices are prepared by first activating with reactive chemical compounds toward one or more of these functional groups. These chemical groups should be easily activated, such as primary amines, sulfhydryls, aldehydes, carboxylic acids, etc. The activated matrices then can conjugate antigens through a covalent linkage, resulting in ligand immobilization. Bioclone offers different high-quality preactivated chromatography matrices for antigen immobilization.
Learn More
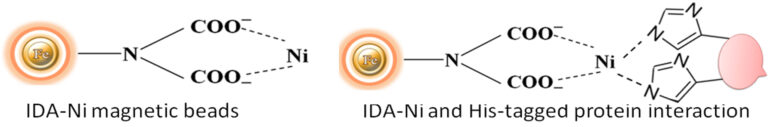
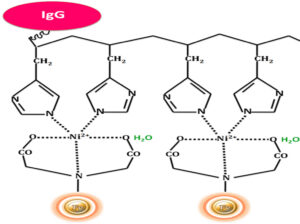
Interestingly, mammalian IgGs contain histidine clusters in their Fc region and can bind to immobilized nickel support (Fig.4), such as BcMag™ IDA Magnetic Beads. Thus, this method can be used to purify and isolate IgGs from a mixture. IgG antibodies can be easily captured from a mixture and gently eluted by optimized conditions. If the binding conditions are not rigorous enough (e.g., using imidazole) and the immunoglobulins are numerous relative to the His-tagged proteins of interest, high background and false positives can occur. Albumins with numerous histidines, such as bovine serum albumin (BSA), can bind to IMAC supports in the absence of His-tagged proteins in the sample or imidazole in the binding/wash buffer.
Explore Products
Ion exchange chromatography (IEC) is one of the most common methods to purify antibodies from different sources and species. It is a handy tool for selective enrichment or specific isolation of mAb or antibodies that do not bind to protein A. The isolation or separation of biological compounds in IEC is based on their reversible adsorption (surface absorption) of charged molecules onto a stationary phase in a given buffer system (pH) (Fig.5). Especially in commercial large scales production of monoclonal antibodies, the optimized conditions in IEC for Binding and releasing the target antibody with a high degree of specificity can apply to nearly all other sample components except antibodies. Consequently, IEC has become a cost-effective, gentle, and reliable technique for antibody purification. For example, anion exchange (AEX) magnetic beads are used to purify mAb; since the isoelectric point (PI) of the mAb is usually high, the mAb molecules do not bind to the beads to pass through the beads when the buffer solution is within pH of 7 ~ 7.5. At the same time, impurities, such as DNA and host cell proteins, are negatively charged in this pH range and bind strongly with the beads to remain on beads.
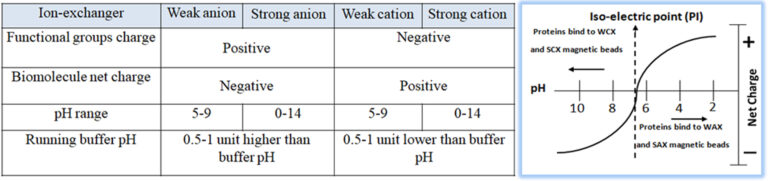
Bioclone offers different high-quality ion exchange matrices for antibody purification.
Select different products –
Beads
Feature
●
A Strong Anion Exchange (SAX) Beads, pKa =~12
●
Used to extract weaker cations that may not bind to WAX Magnetic Beads
●
A Weak Anion Exchange (WAX) Beads, pKa=~11.5
●
Used to extract very strong cations that may be retained irreversibly to SAX Magnetic Beads
●
A Weak Cation Exchange (WCX) Beads, pKa =~4.7
●
Used to extract very strong Anions that may be retained irreversibly to SCX Magnetic Beads
●
A Strong Cation Exchange (SCX) Beads, pKa =~2.3
●
Used to extract weaker Anions that may not bind to WCX Magnetic Beads
●
A Mixed-mode Ion Exchange Magnetic Beads
●
Hydroxyapatite has two adsorbing sites; a calcium site that binds with the acidic groups and a phosphate site that interacts with basic protein groups.
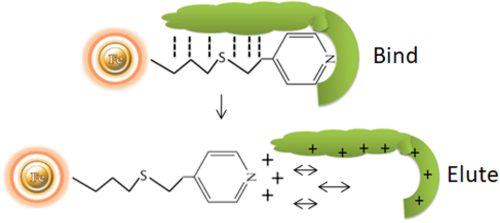
HCIC is a new chromatographic technology for separating antibodies based on the pH-dependent behavior of ionizable, dual-mode ligands. HCIC can efficiently capture and purify antibodies from several sources, including animal serum, ascites fluid, and a variety of cell cultures. Protein-free, chemically defined, protein-supplemented, and serum-supplemented media are all supernatants. Antibody capture settings are consistent with crude samples regarding pH, conductivity, binding capacity, and expression level. The final purity of the antibody is feedstock dependent. However, it can attain degrees of purity as high as 98 percent.
Principle
The separation of antibodies from various biomasses can be accomplished using hydrophobic charge induction chromatography with 4-mercapto-ethyl-pyridine as the ligand. Binding is based on mild hydrophobic contact. It is performed in near-physiological circumstances, without the use of salts. When the pH is reduced, the ligand and the antibody gain a net positive charge under mild acidic circumstances (pH 4.0-4.5).
Bioclone develops a great antibody affinity chromatography matrices – Protein A, G, A/G, and L magnetic beads – used for antibody purification from serum, cell culture supernatant, or ascites, as well as antigen IP/Co-IP from cell or tissue extracts. The procedure for those Magnetic Beads has been improved to allow maximum recovery and purity of the recovered antibody or antigen. For antibody purification, the beads are incubated with the antibody solution, which is magnetically separated from the supernatant. For immunoprecipitation, the beads are delivered to an antigen-containing sample to which an antibody has been introduced and allowed to incubate to form the antibody-antigen complex. The attached antibodies or antigens are dissociated from the beads using an elution buffer and recovered from the solution manually using a magnetic rack or by using automation instruments.
HCIC has several process advantages over more traditional antibody purification methods, such as protein A or ion exchange:
●
Sample preparation is reduced to clarification since feedstocks may be applied without ionic strength or pH adjustment.
●
Preconcentration of dilute samples is unnecessary because effective capture is accomplished even with feedstocks as dilute as 40µg of antibody per ml.
●
Gentle, pH-controlled elution with dilute buffer lowers the possibility of antibody aggregation at lower pH and avoids the requirement for IgG desalting or diafiltration.
Workflow
The separation of antibodies or antibodies present in complex mixtures using chromatography involves the antibodies binding the beads. After Binding, the beads are washed to remove non-antibody protein. Finally, the antibody was eluted from the beads.
Explore Products
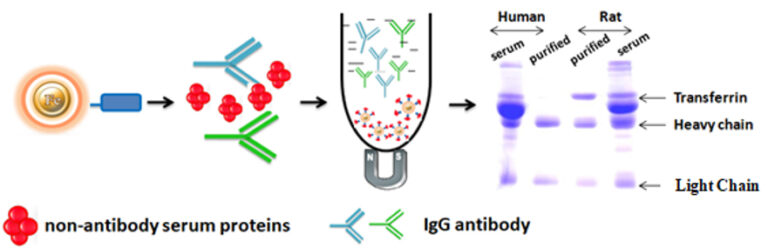
BcMag™ One-Step Antibody Purification Kit is specifically designed for rapid small scale high-throughput purification of a variety of IgG species from serums such as humans, mice, rats, rabbits, goats, horses, guinea pigs, pigs, hamsters, and donkeys. Unlike traditional bind-wash-elute affinity procedures, BcMag™ One-minute Antibody Purification Kit separates antibodies from serum by eliminating non-relevant proteins frequently present in large concentrations. The One-minute Antibody Purification beads bind non-antibody serum proteins such as albumin and transferrin, allowing the antibody to flow through in a moderate buffer ideal for storage and downstream uses. If the serum sample is not hemolyzed, it can be applied straight to the beads without the necessity for ammonium sulfate precipitation.
The One-Step Antibody Purification kit addresses the shortcomings of the commonly utilized immobilized Protein A and Protein G purification methods. IgG species and subclasses are selectively bound using Protein A and Protein G affinity techniques. The technique is often time-consuming and labor-intensive, requiring harsh elution conditions to disrupt the affinity connection. The purified antibody frequently requires dialysis or desalting before storage or use in downstream applications. The One-minute Antibody Purification technique does not require an elution step and instead employs a moderate purification buffer at physiological pH. Furthermore, because the purified antibody is a buffer-free of primary amines, it can be used directly in amine-reactive conjugation chemistries.
Workflow
1.
Add magnetic beads to the serum
2.
Mix the sample by pipetting up and down 25 times (one minute) or by vortex mixer
3.
Magnetic separation of the non-antibody serum protein-bound beads from the pure antibody.
4.
Transfer the supernatant to a fresh tube.
Explore Products
Hydrophobic interaction chromatography (HIC) is a technique for separating macromolecules from one another based on a reversible interaction between the external hydrophobic region of a biological macromolecule and the hydrophobic ligand (such as phenyl, octyl, or butyl) of a HIC medium. The interaction is enhanced by a buffer with a high salt concentration and reduced with a low salt concentration. Therefore, based on salt concentration in a buffer, the protein with less hydrophobicity is eluted first, whereas the protein with more hydrophobicity elutes last. HIC chromatography is often used as a polishing step in monoclonal antibody purification. Compared with other chromatography methods, HIC is a more popular method for separating and purifying protein and peptides in analytical and preparatory scale applications since it employs a less denaturing environment.
Learn More
After antibody purification, removal of contaminant DNA, detergents, and lipids from the solution are necessary for particular applications. Purifying a single target molecule requires many time-consuming washes and spin steps, a lot of hand-on tube handling, and minimal downtime. In contrast, negative chromatography can remove impurities in a single step. In negative chromatography, impurities bind to the adsorbent, and the product flows through the chromatographic column.
Learn More
Magnetic Beads Make Things Simple