- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

A polyhistidine tag, also called 6xHis-tag, His-tagged, and His-tag is a versatile tool that purifies highly pure recombinant protein from various expression systems, including bacterial, yeast, plant cell, and mammalian cells systems. The tag consists of six or more histidine residues positioned at either N or C terminus of a recombinant protein. Due to its small size, His-tag has several distinctive features, including less immunogenicity, hydrophilic nature, and versatility under native and denaturing conditions. Additionally, it is unnecessary to cleave the tag from the recombinant protein since it rarely perturbs the structure and function of its fusion protein. The purification principle of the His-tag is based on immobilized metal ion affinity chromatography (IMAC).
Immobilized metal ion affinity chromatography (IMAC) is a rapid affinity purification chromatography. The proteins or peptides are separated based on their affinity for divalent metal ions chelated to a solid matrix such as beaded agarose or magnetic beads (Fig1.). At pH 7-8, protein or peptide containing consecutive amino acids such as histidine, tryptophan, and cysteine will bind to the chelated metal ions (e.g., Zn2+, Cu2+, Cd2+, Hg2+, Co2+, Ni2+, and Fe2+). Iminodiacetate (IDA) and nitrilotriacetic acid (NTA) are commonly chelating groups, and Ni2+ and Co2+ are the mainly used ions. The structure of the ligand IDA is shown in Figure 1. The binding reaction with the target protein is affected by various independent variables such as pH, temperature, salt type, salt concentration, immobilized metal and chelate ligand density, and protein size. The bound protein is eluted by a decreasing pH gradient, by an increasing imidazole concentration, or by adding an EDTA chelator in a buffer. This technique is an ideal tool for capturing and purification of his-tagged recombinant protein in a quick, inexpensive, and straightforward manner.
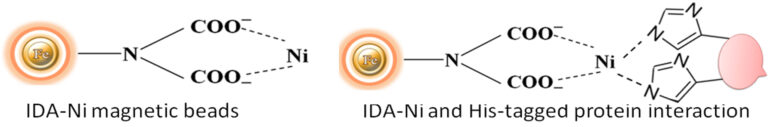
Although IMAC is a very effective protein purification technique, they are mainly made from the traditional affinity chromatography matrices such as agarose resin or column. The problems with their procedures are tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. We developed an extremely efficient magnetic IMAC separation system to overcome these limitations.
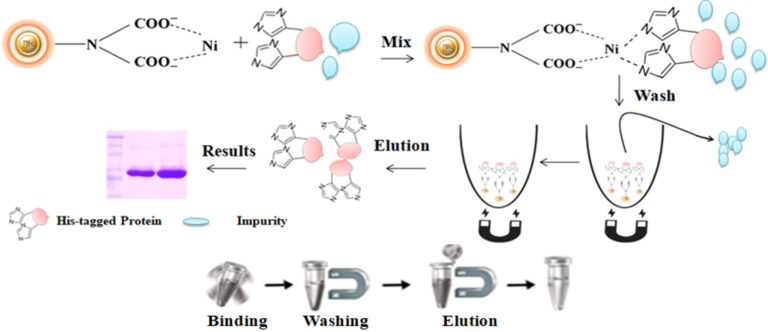
Magnetic beads (particles) are an entirely different type of solid support matrices from beaded agarose or other porous resins. They are much smaller (typically 1-5 µm diameter), thus providing larger surface areas for a high density of ligand immobilization. The beads are manufactured using nanometer-scale superparamagnetic iron oxide as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide. The pure inert silica makes less nonspecific binding. The purification with magnetic beads is straightforward. Mix the magnetic beads with the sample and incubate with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure his tagged recombinant proteins are eluted by imidazole.
Advantages of magnetic particles over other matrices
●
Magnetic beads exhibit less nonspecific binding than porous supports.
●
Stable covalent bond with minimal ligand leakage
●
Direct purification from cell lysate without performing sample treatment.
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High-throughput: Compatible with many different automated liquid handling systems.
Workflow
1.
Harvest the cell by centrifuge
2.
3.
Mix the beads with the cell lysates.
4.
Wash the beads to remove unbound molecules.
5.
Elute the His-tagged protein by imidazole or EDTA containing buffer or by the acid buffer.
Explore
1.
Jansen, J-C. (Editor). (2011). Protein Purification: Principles, High-Resolution Methods, and Applications. 3rd edition. Volume 151 of Methods of Biochemical Analysis. John Wiley & Sons, Hoboken, NJ
2.
Bornhorst, J.A. and Falke, J.J. (2000). Purification of Proteins Using Polyhistidine Affinity Tags. Methods Enzymol. 326: 245-254.
3.
Spriestersbach A, Kubicek J, Schäfer F, Block H, Maertens B. Purification of His-Tagged Proteins. Methods Enzymol. 2015;559:1-15. doi: 10.1016/bs.mie.2014.11.003. Epub 2015 May 4. PMID: 26096499.
4.
Zhang C, Fredericks D, Longford D, Campi E, Sawford T, Hearn MT. Changed loading conditions and lysate composition improve the purity of tagged recombinant proteins with tacn-based IMAC adsorbents. Biotechnol J. 2015 Mar;10(3):480-9. doi: 10.1002/biot.201400463. Epub 2014 Oct 31. PMID: 25303209.
5.
Pina AS, et al. (2014) Affinity tags in protein purification and peptide enrichment: An overview. Methods in molecular biology (Clifton, N.J.) 1129: 147-168.
6.
Young CL, et al. (2012) Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol J 7(5): 620-634.
Magnetic Beads Make Things Simple