- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
Specification
Composition
Magnetization
~60 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.5 g/ml
Concentration
Binding Capacity
>2mg His-tagged GFP /ml of Beads
Storage
Store at 4ºC upon receipt
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit at 4ºC on arrival.
The purification with magnetic microparticles is straightforward. Mix the microparticles with the cell lysate and incubate them with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure His-tagged recombinant proteins are eluted by imidazole.
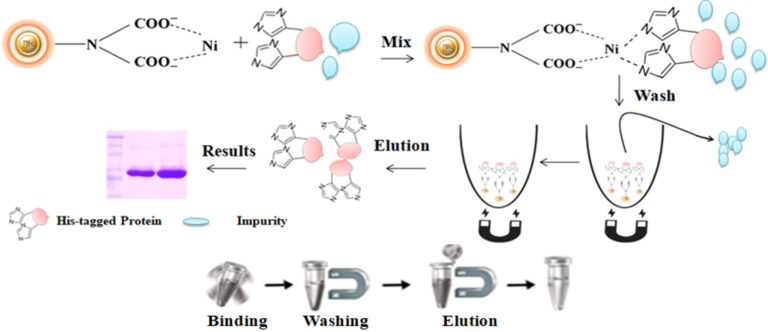
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Stable covalent bond with minimal ligand leakage
●
High binding capacity, very low nonspecific binding
●
Scalable – Easily adjusts for sample size and automation
●
Reproducible results
●
Investigating protein structure and function
●
Preparing recombinant proteins for X-ray crystallography
●
Ideal for study of protein interactions with protein or DNA
●
Immunization to raise antibodies against a protein of interest
●
Effective screening protein expression even with crude cell lysates
●
Microscale purification of his-tagged proteins.
A polyhistidine tag, also called 6xHis-tag, His-tagged, and His-tag, is a versatile tool for purifying the highly purified recombinant protein from various expression systems, including bacterial, yeast, plant cell, and mammalian cells systems. The tag comprises six or more consecutive histidine amino acid residues positioned at either N or C terminus of a recombinant protein. Due to its small size, His-tag has several distinctive features, including less immunogenicity, hydrophilic nature, and versatility under native and denaturing conditions. Additionally, it is unnecessary to cleave the tag from the recombinant protein since it rarely perturbs the structure and function of its fusion protein. The purification principle of the His-tag depends on immobilized metal ion affinity chromatography (IMAC).
Although IMAC is a very effective protein purification technique, they are mainly based on traditional affinity chromatography matrices such as agarose resin or column. These solid matrices make the purification process tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. We developed an extremely efficient magnetic IMAC separation system to overcome these limitations.
1.
Jansen, J-C. (Editor). (2011). Protein Purification: Principles, High-Resolution Methods, and Applications. 3rd edition. Volume 151 of Methods of Biochemical Analysis. John Wiley & Sons, Hoboken, NJ
2.
Bornhorst, J.A. and Falke, J.J. (2000). Purification of Proteins Using Polyhistidine Affinity Tags. Methods Enzymol. 326: 245-254.
3.
Spriestersbach A, Kubicek J, Schäfer F, Block H, Maertens B. Purification of His-Tagged Proteins. Methods Enzymol. 2015;559:1-15. doi: 10.1016/bs.mie.2014.11.003. Epub 2015 May 4. PMID: 26096499.
4.
Zhang C, Fredericks D, Longford D, Campi E, Sawford T, Hearn MT. Changed loading conditions and lysate composition improve the purity of tagged recombinant proteins with tacn-based IMAC adsorbents. Biotechnol J. 2015 Mar;10(3):480-9. doi: 10.1002/biot.201400463. Epub 2014 Oct 31. PMID: 25303209.
5.
Pina AS, et al. (2014) Affinity tags in protein purification and peptide enrichment: An overview. Methods in molecular biology (Clifton, N.J.) 1129: 147-168.
6.
Young CL, et al. (2012) Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol J 7(5): 620-634.
Magnetic Beads Make Things Simple