- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]
The technique of covalently attaching a fluorophore to another molecule, such as a protein or nucleic acid, is known as fluorescent labeling. The availability of novel fluorophores has increased the possibilities for sensitive detection of biomolecules and investigation of their interactions substantially. Improved fluorescent dyes have made possible previously unattainable research of cellular structures and processes. Fluorescent labels have numerous advantages, including the fact that they are highly sensitive even at low concentrations, persistent over long periods, and do not interfere with the function of the target molecules. Labeled cells can be tracked in vitro and in vivo using targeted imaging. Using various fluorophores in the same sample allows for the simultaneous observation of many molecules. Fluorescein IsoThioCyanate (FITC), rhodamine derivatives (TRITC), coumarin, and cyanine are the most prevalent fluorophores. These synthetic organic dyes are used to label biomolecules such as proteins, peptides, antibodies, nucleic acids, bacteria, and yeast. Naturally occurring fluorochromes, such as Green Fluorescent Protein (GFP), can also be utilized to mark living cells genetically.
Labeling molecules necessitates the use of a chemically reactive fluorophore derivative. Common reactive groups include amine-reactive isothiocyanate derivatives such as FITC, amine-reactive succinimidyl esters such as NHS-fluorescein or NHS-rhodamine, and sulfhydryl-reactive maleimide-activated fluors such as Fluorescein-5-maleimide. When any of these reactive dyes react with another molecule, a stable covalent connection is created between the fluorophore and the tagged molecule. Isothiocyanates have long been utilized as primary reactive chemistry for binding fluorescent dyes to proteins via the primary amines of lysine side chains. NHS-ester chemistry is presently the preferred labeling method because it has a higher specificity for primary amines and results in a more stable bond after labeling. Sulfhydryl-reactive chemicals have a narrower application in protein labeling when lysine residues must be conserved, or a cysteine residue is particularly targeted to localize the fluorescent dye on the labeled protein.
Fluorescence measurement can be divided into two categories: steady-state fluorescence and time-resolved fluorescence. TRF is quite similar to ordinary fluorometric detection. The primary distinction between the two measures is the timing of the excitation/emission process. Excitation and emission are simultaneous during typical fluorometric detection; the light emitted by the sample is measured while Excitation is occurring. On the other hand, TRF relies on the employment of very particular fluorescent molecules known as lanthanide chelate labels, which allow detection of the produced light to occur after Excitation. The europium ion (Eu3+) is the most frequent lanthanide chelate label.
Förster resonance energy transfer (FRET) is based on the transfer of energy between two fluorophores in close proximity, a donor (long-lived fluorescence) and an acceptor (short-lived fluorescence). The level of energy transfer between biomolecules can be measured by labeling each partner with a fluorescent marker and measuring the level of interaction. Organic luminous chemicals such as fluorescein and rhodamine were once commonly utilized in fluorescence assays. However, these bioassays have significant disadvantages in that fluorescent detection is significantly inhibited by noise in the background derived from scattered excitation light and interfered with by fluorescence from coexisting material in the sample (fluorescent compounds and dust/line) making obtaining a highly sensitive measurement difficult.
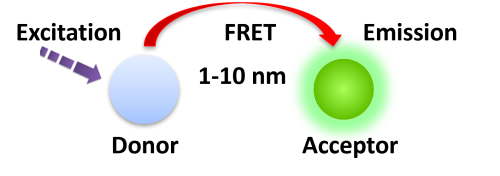
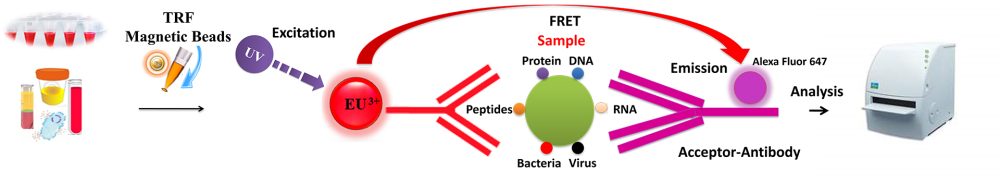
BcMag™ TR-FRET Assay, in contrast to typical FRET assays, uses time-resolved fluorescent magnetic beads (BcMag™ TR-Magnetic Beads) as the donor fluorophore. The donor and acceptor can be two proteins, two DNA strands, an antigen and an antibody, or a ligand and its receptor. After a reasonable time delay (usually 50 to 100 s), a signal is generated by fluorescence resonance energy transfer between a donor and an acceptor molecule when they are close and monitored in a time-resolved way. In BcMag™ TR-FRET Assay, a trace amount of analytes can be easily enriched from the complex by TR-Magnetic Beads, resulting in higher sensitivity. This Assay practically eliminates all fluorescence backgrounds caused by the sample and plastic microplate, as well as by direct acceptor excitation. As a result, the signal-to-noise ratios of the BcMag™ TR-FRET Assay are very high, and the background is quite low. Furthermore, the Assay does not need washing steps. BcMag™ TR-FRET Assay offers substantial advantages to bioassays in high throughput screening, such as assay flexibility, dependability, increased assay sensitivity, higher throughput, and fewer false positive/false negative results.
Magnetic bead separation is a fast, effective, and clean method used by scientists to replace filtering, centrifugation, and separation processes. Time-resolved Fluorescence Magnetic Beads can be used for immunoassays and other applications. They have high surface-to-volume ratios, small sizes (0.1-10µm), various functional groups attached to the surfaces (e.g., antibodies, DNA, and chemical groups), and the ability to manipulate the particles via an applied magnetic field easily. Combined with automated liquid handling and robust detection instrumentation, these characteristics enable a wide range of high-throughput applications.
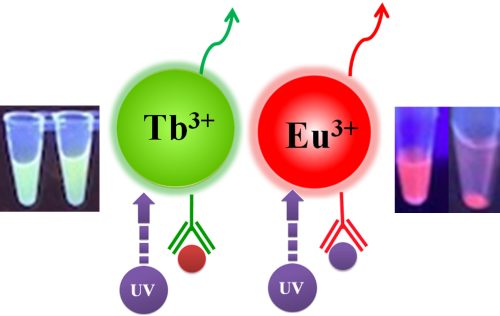
Table 1. Fluorescence Properties of TRF Magnetic Beads
Fluorophore
Red
Green
Far-Red
340
320
470
615
545
710
Fluorescence lifetime (Ԏ) (µsec)
730
1050
354.36
Stokes shifts
(nm)
275
220
175
Fluorophore
Fluorescence lifetime (Ԏ) (µsec)
Stokes shifts
(nm)
Red
340
615
730
275
Green
320
545
1050
220
Far-Red
470
710
354.36
175
1.
Perform a double function simultaneously on the same beads: The magnetic beads combine separation/preconcentration and detect analytes, allowing quick, simple, robust, and high-throughput analytes of trace amounts from complex biological samples on the same beads.
2.
Ultra sensitive. Lower detection limits of 10 pg/mL versus typical fluorometric detection limits of 100 pg/mL.
3.
Extremely photostable and highly resistant to photobleaching. All the lanthanides chelate or cryptate molecules and iron oxide are entirely encapsulated inside each bead instead of merely on the bead’s surface. The protective environment prevents iron oxide and dye from leaching into aqueous media, which makes the beads less sensitive to external conditions such as solvent, temperature, pH, etc.
4.
Very high fluorescent intensity. Because a single bead has a large concentration of lanthanide chelate with a high quantum yield ranging from 40 to 90%, the beads show excellent fluorescence intensity, which increases test sensitivity without signal amplification. Such bright beads are also perfect for donors’ use in time-resolved FRET assays.
5.
Lanthanides chelate or cryptate has large Stokes shifts (>250 nm), narrow emission bands (-10 nm bandwidth), and long fluorescence lifetime (μs), which dramatically reduces background and increases the signal-to-noise ratio.
6.
Most bioprocess ELISA assays can be converted to an HTRF assay.
7.
No washing step is involved in the assays.
8.
Have a hydrophilic silica surface grafted by different functional groups with linkers of variable lengths, allowing efficient conjugation of various ligands such as peptides, proteins, antibodies, small molecules, carbohydrates, aptamers, DNA/RNA, etc.
9.
Due to the microsphere’s magnetic property, the fluorescent magnetic beads are suitable for high-throughput automation.
The TRF beads assay is straightforward.
1.
Mix the antibody-conjugated donor beads with the cell lysates and incubate them with continuous rotation for a sufficient time. The beads remain suspended in the sample solution during mixing, allowing the target analytes to bind to the donor beads.
2.
After incubation, the beads are collected and separated from the sample using a magnet rack.
3.
Add the antibody-conjugated acceptor and incubate them with continuous rotation for a sufficient time.
4.
Analysis of numerous microplate readers supports TR-FRET measurements.
Selection of TRF magnetic beads and acceptor
Table 2. Selection of TRF Magnetic Beads and Acceptor
TRF-Magnetic Beads
Excitation
Emission
Acceptor
BcMag™ Europium Fluorescent Magnetic Beads
340 nm
615 nm
BcMag™ Europium Fluorescent Magnetic Beads
340 nm
615 nm
AlexaFluor 647
BcMag™ Europium Fluorescent Magnetic Beads
340 nm
615 nm
XL665/d2
BcMag™ Terbium Fluorescent Magnetic Beads
320 nm
545 nm
Fluorescein / GFP
BcMag™ Ruthenium Fluorescent Magnetic Beads
470 nm
710 nm
Far-red Dye
Learn More
Making ligand-specific magnetic beads used in TR-FRET Assay requires methods to covalently conjugate ligands (protein, DNA and etc) to appropriate TR magnetic beads and acceptor dye. Immobilization and conjugation refer to the covalent attachment of ligands to magnetic beads and acceptor dye through their common chemical groups. This method prepares the matrices by activating with reactive chemical compounds toward one or more of these functional groups. These chemical groups should be easily activated, such as primary amines, sulfhydryls, aldehydes, carboxylic acids, etc. The activated matrices then can conjugate antigens through a covalent linkage, resulting in ligand immobilization.
The type of linkage created between the matrix and the immobilized ligand influences the bead’s performance in a variety of ways. For example, if the linkage obstructs or negatively influences the structure of the immobilized ligand, its efficiency for TR-FRET Assay will be limited.
Immobilization chemistry
The following general considerations apply to creating a successful magnetic affinity support matrix.
●
Linkers/spacers: Linkers are flexible molecules or stretch of molecules that link two molecules of interest together. The linkers’ property affects the affinity support’s performance in several ways.
●
Linker length. Suppose ligands are small peptide antigens or molecular. In that case, it is best to use a long arm linker (>10 atoms) attached to the support matrix since it can avoid the steric hindrance (The ligand is inaccessible to the binding site) (Fig.4). Linker length is less critical for large (e.g., protein) antigens since the antigen itself is an effective linker between the support matrix and the epitope.
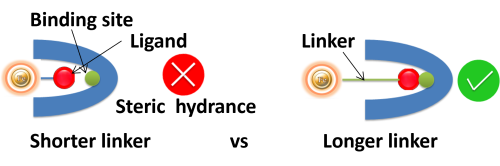
●
Site-directed immobilization: Using a unique functional group for site-directed immobilization is beneficial for correct orientation, such as sulfhydryl on a single terminal cysteine in a peptide for a small antigen) and carbohydrate residues on the Fc side for antibody.
●
Use hydrophilic linker to immobilize antigen to activated support to reduce background noise.
●
Use a cleavable linker to separate the target from the solid matrix easily.
Immobilization chemistries
Affinity ligands can be linked (immobilized) to the solid substance (stationary phase) in various ways.
The amine group (-NH2) is the most common functional target for immobilizing protein molecules: This group can be found at the N-terminus of each polypeptide chain (called the alpha-amine) and in the side chain of lysine (Lys, K) residues (called the epsilon-amine). Because of their positive charge under physiological conditions, primary amines are frequently outward-facing (i.e., on the outside surface) of proteins; hence, they are usually available for conjugation without denaturing the protein structure.
BcMag™ NHS-Activated TR Magnetic Beads are uniform, magnetic beads coated with high-density NHS (N-hydroxyl succinimide) functional groups on the surface. The magnetic resin utilizes reliable NHS-ester chemistry and does not require the use of dangerous chemicals for immobilization. The beads have less nonspecific binding and no leaking of the coupled ligand. The resin can quickly, efficiently, and covalently conjugate any primary amine-containing ligands by forming stable amide linkages (Fig.5) with better than 85% coupling efficiency. Bioclone NHS-Activated beads reactions take less than an hour (15–30 min at room temp pH 7–9, 4 hours at 4°C) and produce substantially more stable linkages.
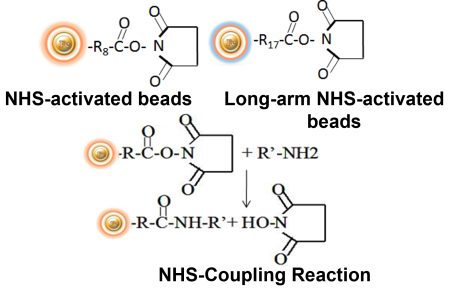
Features and Advantages
●
Pre-activated and ready-to-use
●
Easy to use
●
A quick coupling in pH 7.4 at 4°C to 25°C
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable immunoaffinity matrices
●
Low nonspecific binding
●
Immobilize 1-10 mg protein or 0.1-1 mg peptide/ml beads
●
Applications: Cell sorting, Immunoprecipitation; Purification for Antibodies, Proteins/Peptides, DNA/RNA
Explore Products
Labeling Protocol
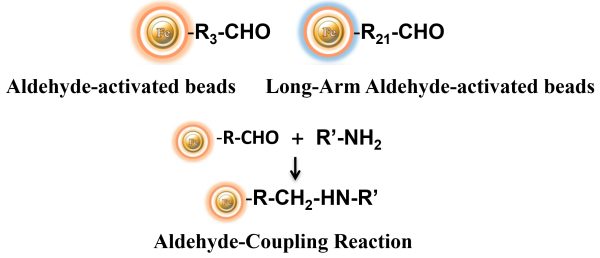
BcMag™ Aldehyde-Activated TR Magnetic Beads are suitable for conjugating a large protein. The Aldehyde-Activated resins have higher coupling efficiency than cyanogen bromide (CNBr) activated supports. Furthermore, the aldehyde Chemistry generates an uncharged connection with the amine-containing ligand that is more stable than the CNBr approach. The hydrophilic surface ensures excellent dispersion and easy handling in various buffers. When utilized for affinity purification methods, these features allow better leak-resistant immobilization and lower nonspecific binding.
Features and Advantages
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Stable covalent bond with minimal ligand leakage
●
High capacity – Immobilize 15 -20 µg antibody/mg beads
●
Scalable – easily adjusts for sample size and automation
●
Low nonspecific binding
●
Reproducible results
●
Application: Purification for antibody, protein/peptide, DNA/RNA, cell sorting, Immunoprecipitation
Explore Products
Labeling Protocol
It is frequently helpful to immobilize affinity ligands via functional groups other than amines. The thiol group, in particular, can be employed to guide coupling processes away from active centers or binding sites on specific protein molecules. Sulfhydryls, commonly known as thiols, are found in proteins as side chains of cysteine (Cys, C) amino acids. As the foundation of native tertiary or quaternary protein structure, pairs of cysteine sulfhydryl groups are frequently linked via disulfide bonds (-S-S-) within or between polypeptide chains. For most thiol-reactive compounds, only free or reduced sulfhydryl groups (-SH) [rather than sulfur atoms in disulfide bonds] are available for reaction.
Advantages of protein conjugation via sulfhydryl groups:
First, sulfhydryls are found in most proteins but are not as abundant as primary amines, immobilizing via sulfhydryl groups more selective and precise. Second, because sulfhydryl groups in proteins are frequently engaged in disulfide bonds, crosslinking at these locations usually has little effect on the underlying protein structure or blocks binding sites. Third, the quantity of accessible (i.e., free) sulfhydryl groups can be easily regulated or manipulated; they can be formed by reducing native disulfide bonds or introduced into molecules via reactivity with primary amines. Finally, combining sulfhydryl-reactive groups with amine-reactive groups to create heterobifunctional crosslinkers allows for more flexibility and control over crosslinking methods.
BcMag™ Iodoacetyl-activated TR Magnetic Beads are uniform, silica-based superparamagnetic beads coated with a high density of Iodoacetyl functional groups on the surface. It is designed to enable fast, efficient, and covalent immobilization of protein, peptides, and other ligands through their sulfhydryl groups (-SH) for affinity purification procedures. At physiological to alkaline circumstances (pH 7.2 to 9) in either aqueous or organic solvents 20- 30% DMSO or DMF, iodoacetyl-activated supports react with sulfhydryl groups, resulting in stable thioether bonds. These reactions are often carried out in the dark to prevent the formation of free iodine, which can react with tyrosine, histidine, and tryptophan residues. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
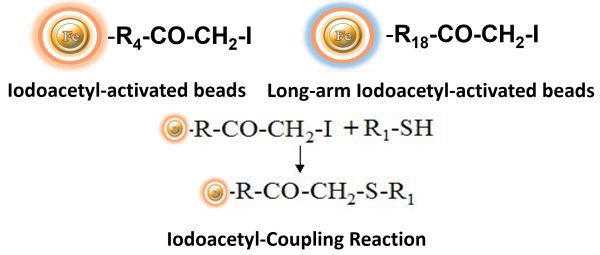
Features and Benefits
●
Iodoacetyl groups react selectively with sulfhydryl (-SH) groups to produce irreversible thioether linkages.
●
Fast – couple peptide samples in 2 hours.
●
A cleavable built-in disulfide bond allows the ligand-target molecule complex to separate from the beads.
●
Versatile coupling conditions – as needed for protein or peptide solubility during the coupling reaction, employ pH 7.5 to 9.0 aqueous buffers, organic solvent (e.g., 20% DMSO), or denaturant (guanidine HCl).
●
Simple to follow protocols for sample preparation, immobilization, and affinity purification
●
High capacity – Immobilize 15 -20 µg antibody/mg beads.
Applications
●
Immobilize peptides with terminal cysteine residues to purify antibodies raised against peptide immunogens.
●
Immobilize antibodies in an orientated manner using hinge-region sulfhydryls to ensure that antigen binding sites are not sterically inhibited when antigen affinity purification is performed.
●
Produces reusable immunoaffinity matrices
●
Maintains antibody function – immobilizes IgG via the Fc region, leaving both antigen binding sites available for target capture.
Explore Products
Labeling Protocol
In their natural condition, most biological compounds do not contain carbonyl ketones or aldehydes. However, similar groups on proteins may be effective for forming an immobilization site that drives covalent coupling away from active centers or binding regions. Glycoconjugates, such as glycoproteins and glycolipids, typically contain sugar residues with hydroxyls on neighboring carbon atoms; these cis-diols can be oxidized with sodium periodate to produce aldehydes as covalent immobilization sites.
BcMag™ Hydrazide Magnetic Beads are uniform magnetic beads grafted with a high density of hydrazide functional groups on the surface. Hydrazide chemistry is effective for labeling, immobilizing, or conjugating glycoproteins via glycosylation sites, which are frequently (as with most polyclonal antibodies) positioned in domains distant from the critical binding sites whose function needs to be preserved. Coupling antibodies in this manner selectively targets heavy chains in the Fc portion of the molecule, assisting in the best possible preservation of antigen binding activity by the ends of the Fv regions. At pH 5 to 7, hydrazide-terminated supports and compounds will conjugate with carbonyls of oxidized carbohydrates (sugars), resulting in the production of hydrazone linkages.
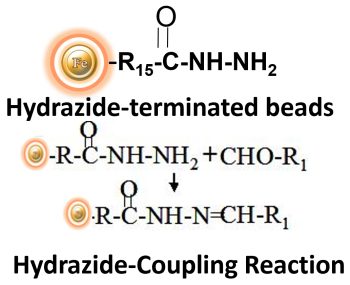
Features and Benefits
●
Specific immobilization—the hydrazide-activated beads bind exclusively to pure glycoproteins containing sugar groups that have been gently oxidized with periodate (e.g., sialic acid).
●
Stable covalent bond with low levels of ligand leakage
●
Maintains antibody function—immobilizes IgG via the Fc region, leaving both antigen binding sites available for target capture.
●
Low nonspecific binding
●
High capacity – Immobilize 15-20µg antibody/mg beads.
Explore Products
Conjugation Protocol
The carboxyl group is found in a wide range of biological substances. Peptides and proteins contain carboxyls (-COOH) at the C-terminus of each polypeptide chain as well as aspartic acid (Asp, D) and glutamic acid side chains (Glu, E). Carboxyls, like primary amines, are typically found on the surface of protein structures.
Carboxylic acids can be employed to immobilize biological molecules by applying a carbodiimide-mediated process. Although no activated support has a reactive group that is spontaneously reactive with carboxylates, chromatographic supports containing amines (or hydrazides) can be utilized to create amide bonds with carboxylates activated using the water-soluble carbodiimide crosslinker EDC.
BcMag™ Amine-activate TR Magnetic Beads are magnetic beads coated with a high density of primary amine functional groups on the surface. The beads can covalently conjugate primary amine or carboxy-containing ligands such as protein and peptides for affinity purification (Fig.9). The hydrophilic surface ensures beads have low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
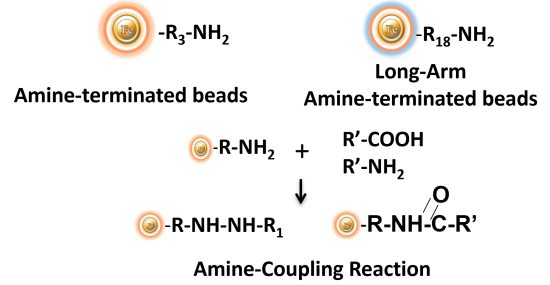
Features and Benefits
●
Covalently coupled with high efficiency
●
Easy to use
●
Stable covalent bond with low levels of ligand leakage
●
Produces reusable immunoaffinity matrix
●
Low nonspecific binding
●
Immobilize 15-20 µg antibody/mg beads
●
A cleavable built-in disulfide bond allows the ligand-target molecule complex to separate from the beads.
●
Application: Purification for antibody, protein/peptide, DNA/RNA, cell sorting, Immunoprecipitation
Explore Products
Labeling Protocol
BcMag™ Carboxyl-activated TR Magnetic Beads are uniform, silica-based magnetic beads coated with a high density of carboxylic acid groups on the surface. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The high density of pendent functional carboxylic acid groups on their surface allows covalently conjugate primary amine-containing ligands via a stable amide bond (Fig.10). Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
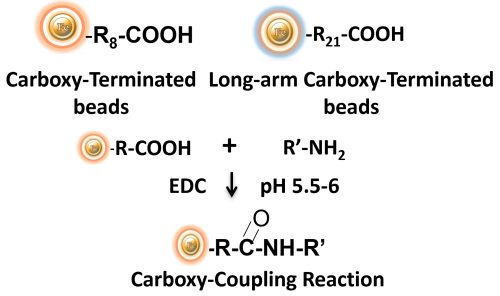
EDC and other carbodiimides are zero-length crosslinkers that cause carboxylates (-COOH) to conjugate directly to primary amines (-NH2) without becoming part of the ultimate crosslink (amide bond) between target molecules. When amine-terminated resin is employed as the primary amine in this process, ligand immobilization occurs.
Because peptides and proteins contain numerous carboxyls and amines, direct EDC-mediated crosslinking typically results in random polypeptide polymerization. However, this reaction chemistry is commonly utilized in immobilization methods (for example, binding proteins to a carboxylated surface) and immunogen production (e.g., attaching a small peptide to a large carrier protein). Indeed, some peptide polymerization is advantageous in antibody manufacturing and purification operations because it boosts overall peptide conjugation (loading) and ensures that the peptide ligands are supplied in numerous orientations.
Explore Products
Labeling Protocol
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.
Magnetic Beads Make Things Simple