- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

All BcMag™ hydrophobic magnetic beads are designed as uniform magnetic beads grafted with a high density of hydrophobic ligands on the surface. Our hydrophobic magnetic resins are rigid polymeric beads with covalent surface chemistries, allowing easier handling and packing while providing more excellent physical and chemical stability—resulting in a robust production process. The magnetic resins replace time-consuming, difficulty, and expensive chromatographic techniques such using agarose-, cellulose-, Sepharose-, sephadex-based columns or resins. The hydrophobic magnetic beads are manufactured using nanometer-scale superparamagnetic iron oxide as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide. The pure inert silica makes less nonspecific binding. The beads are much smaller (1, 2.5, and 5 µm diameter) in size and are non-porous, which exhibit larger surface area, less nonspecific binding, and higher resolution than porous supports.
Hydrophobic interaction chromatography (HIC) is a technique for separating macromolecules from one another based on a reversible interaction between the external hydrophobic region of a biological macromolecule and the hydrophobic ligand (such as phenyl, octyl, or butyl) of a HIC medium (Fig.1). The interaction is enhanced by a buffer with a high salt concentration and reduced with a low salt concentration. Therefore, based on salt concentration in a buffer, the protein with less hydrophobicity is eluted first, whereas the protein with more hydrophobicity elutes last. Compared with other chromatography methods, HIC is a more popular method for separating and purifying protein and peptides in analytical and preparatory scale applications since it employs a less denaturing environment.
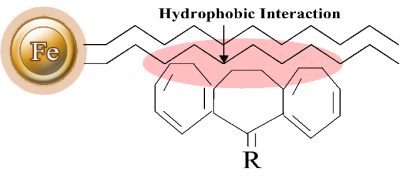
Reverse-phase chromatography (RPC) is also used to separate proteins and peptides based on hydrophobic interaction chromatography. However, the ligands of an RPC medium are more hydrophobic than that of a HIC. This interaction is usually so strong that only organic solvents (e.g., acetonitrile or methanol) can successfully elute the proteins/peptides. Since organic solvents can denature many proteins, RPC is not recommended for protein purification.
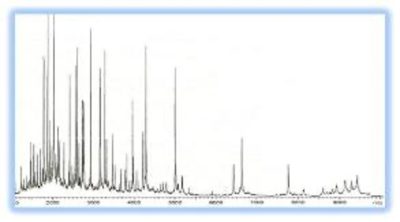
Magnetic resin chromatography is a novel technique that is an excellent alternative to the traditional chromatography methods and has been rapidly adopted in traditional and clinical proteomics. Magnetic separation with those resins has several advantages over standard chromatography or spin columns. Magnetic separation is not only simple, quick, reproducible, and extraordinarily gentle but can also be performed directly in crude samples containing suspended solid material and in one single test tube. The samples include serum, plasma, urine or cell lysates, ascites fluid, whole blood, milk, whey, urine, cultivation media, waste from the food and fermentation industry, etc. It does not need expensive liquid chromatography systems, centrifuges, filters, or other equipment. Due to the magnetic property, it can be easily adopted by various automated systems for large-scale purification. BcMag™ hydrophobic magnetic beads were used in a wide range of applications such as peptide mapping, purity checking, isolation of active biomolecules, separation of drugs and metabolites, and extraction of various contaminants in environmental samples. These resins can be used in aqueous solutions and solvents such as alcohols, petroleum ether, diethyl ether, and hexane, as well as solvent mixes, without the resins expanding or contracting. Figure 1 is a Typical MALDI-TOF MS spectra (1-10 kDa) of LMW serum fraction processed on 1um C18 magnetic beads. (Dr. Radoslav (Rado) Goldman, Georgetown University Medical Center )
●
HIC media consist of alkyl or aryl ligands that are connected to magnetic resins.
●
A relatively high salt buffer is utilized. The typical salts used are 1-2M ammonium sulfate or 3M sodium chloride, which are chosen to increase the essential interaction between the protein sample and the resins while limiting the interaction of other less hydrophobic proteins (impurities).
●
The beads are washed to remove non-bound proteins, and the salt concentration is gradually reduced to begin eluting proteins. Proteins with the lowest hydrophobicity are eluted first when salt gradients are manipulated.
●
A final wash with a salt-free buffer aids in the removal of firmly attached proteins. Additives in the buffer can help the bound proteins desorb even more. Water-miscible alcohols, chaotropic salt solutions, and detergents are examples of additives. In some cases, stronger conditions such as 0.5-1.0M sodium hydroxide, 70% ethanol, or 30% isopropanol are required to eliminate all bound proteins.
●
The type of ligand utilized impacts the adsorption behavior of proteins; for example, straight-chain alkyl ligands are hydrophobic, whereas aryl ligands exhibit aromatic and hydrophobic interactions.
●
Hydrophilic carbohydrates such as cross-linked agarose and synthetic copolymer materials are the most widely utilized supports. Even when using the same ligand, the selectivity of different supports will vary.
●
Degree of substitution – A protein’s binding capability is related to the degree of substitution of the ligand. However, significant quantities of ligand replacement strengthen the binding and make protein elution problematic.
●
The temperature has a direct and positive relationship with the affinity of hydrophobic interactions. Protein structure and solubility are also affected by high temperatures. Temperature is rarely used to control molecule elution in HIC.
●
The mobile phases commonly used in HIC have a neutral pH range of 5 – 7. The impact of pH on protein-medium interactions varies depending on the protein. When the protein charge increases, the hydrophobic interaction between medium and protein diminishes. While pH affects the degree of protein binding, it is not thought to be significant enough to use pH gradients for solute elution.
The salt concentration – Adding salt to the buffer and sample increases ligand-protein interaction; however, protein precipitation occurs at high salt concentrations. Sodium, ammonium, or potassium sulfates have greater precipitation effects, despite being quite effective in enhancing ligand-ligand interaction.
Beads
Feature
●
Suitable for fractionation of larger molecular weight proteins and peptides. Use this less retentive phase to release hydrophobic molecules by weaker organic solvents rapidly.
●
Good matrix for fractionation of very large hydrophobic molecules which are bound too strongly to C-18 beads to be eluted.
●
The least selective phase binds most organic analytes from aqueous matrices.
Beneficial for extracting numerous analytes diverse in structure for the same sample.
●
Ideal for very hydrophobic analytes that may be irreversibly retained on more hydrophobic C-18 beads
●
Similar in polarity to C8 beads. However, an electron-dense aromatic ring offers some unique selectivity and retention.
●
Used for polypeptides with aromatic side chains, large, hydrophobic proteins, membrane-spanning.
●
Peptides, lipid peptides, fusion proteins from inclusion bodies
1.
Aguilar, M. I., and Hearn, M. T. W. (1996) High resolution reversed-phase high performance liquid chromatography of peptides and proteins. Meth. Enzymol.270, 3–26. 2.
2.
Bupp CR, Wirth MJ. Making Sharper Peaks for Reverse-Phase Liquid Chromatography of Proteins. Annu Rev Anal Chem (Palo Alto Calif). 2020 Jun 12;13(1):363-380.
3.
Purcell, A. W., Aguilar, M. I., and Hearn, M. T. W. (1995) Conformational effects in the RP-HPLC of polypeptides. II: The role of insulin A and B chains in the chromatographic behaviour of insulin. J. Chromatogr.711, 71–79. 4.
4.
Josic D, Kovac S. Reversed-phase High Performance Liquid Chromatography of proteins. Curr Protoc Protein Sci. 2010 Aug;Chapter 8:Unit 8.7.
5.
Oroszlan, P., Wicar, S., Teshima, G., Wu, S.-L., Hancock, W. S., and Karger, B. L. (1992) Conformational effects in the reversed-phase chromatographic behaviour of recombinant human growth hormone (rhGH) and N-methionyl recombinant human growth hormone (met-hGH). Anal. Chem.64, 1623–1631. 6.
6.
Lin S. and Karger B. L. (1990) Reversed-phase chromatographic behaviour of proteins in different unfolded states. J. Chromatogr.499, 89–102. 7
7.
Unger, K. K. (1990) Silica as a support, in HPLC of Biological Macromolecules: Methods and Applications (Gooding, K. M. and Regnier, F. E., eds.), Dekker, New York, pp. 3–24. 8.
8.
Cuadros-Rodríguez L, Martín-Torres S, Ortega-Gavilán F, Jiménez-Carvelo AM, López-Ruiz R, Garrido-Frenich A, Bagur-González MG, González-Casado A. Standardization of chromatographic signals – Part II: Expanding instrument-agnostic fingerprints to reverse phase liquid chromatography. J Chromatogr A. 2021 Mar 29;1641:461973.
9.
Zhou, N. E., Mant, C. T., Kirkland J. J., and Hodges R. S. (1991) Comparison of silica-based cyanopropyl and octyl reversed-phase packings for the separation of peptides and proteins. J. Chromatogr.548, 179–193.
Magnetic Beads Make Things Simple