- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]
Ion exchange chromatography (IEX) separates ionizable compounds according to their total charge. Because The charge on the molecule of interest is easily modified by adjusting buffer pH, this technique allows for the separation of similar types of molecules that would be hard to separate using other methods. Ion exchange chromatography is a powerful separation technique used for preparative chromatography and analytical chromatography. It is widely used to separate and purify many charged or ionizable molecules such as proteins, peptides, enzymes, nucleotides, DNA, antibiotics, vitamins, etc.
Ion exchange chromatography is a compelling separation technology utilized not only for preparative chromatography but also for analytical chromatography. However, IEX, like all other chromatographic modes, has some restrictions.
One of the main limitations of ion-exchange chromatography is its buffer requirement. Because binding to IEX resins is based on electrostatic interactions between proteins of interest and the stationary phase, IEX columns must be loaded in low-salt buffers. This restriction may necessitate a buffer exchange step before ion-exchange chromatography in some applications.
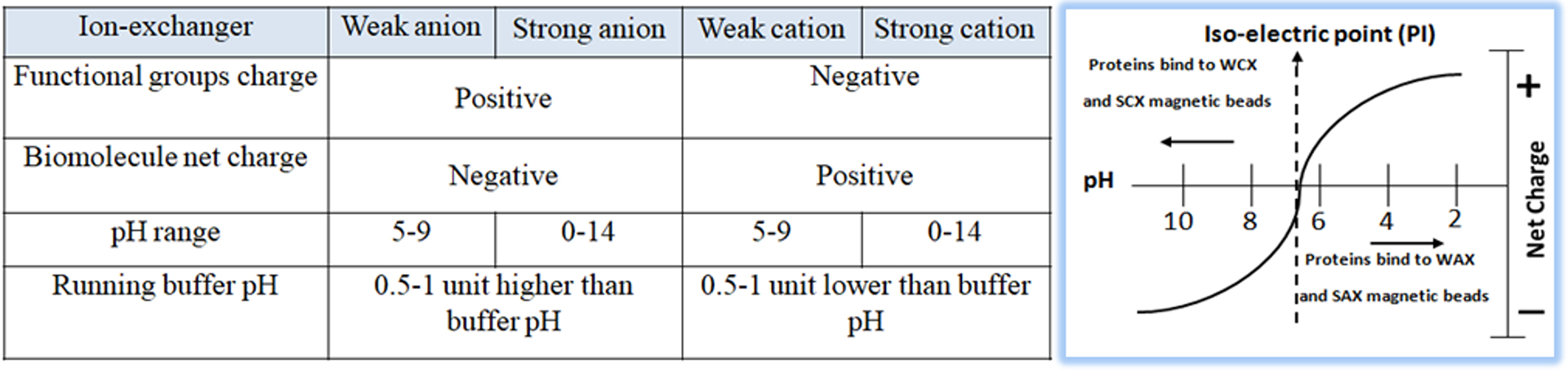
Ion exchange chromatography is classified into two types: anion exchange, in which the chromatography matrix is positively charged, and cation exchange, in which the matrix is negatively charged. Biological molecules such as proteins are zwitterionic molecules with both positively and negatively charged chemical groups. Molecules can have a net positive charge, a net negative charge, or no charge, depending on the pH of their surroundings. The pH where a molecule has no net charge is referred to as the isoelectric point (pI). The protein is positively charged (protonated) in a buffered solution below its pI and will attach to a cation exchange resin’s negatively charged functional groups. In a buffered solution above the protein’s pI, the protein becomes negatively charged (deprotonated) and binds to the positively charged functional groups of an anion exchange resin (Fig.1). To choose the correct buffer for a selected pH, the following is a general rule for selecting a buffer pH:
Anion exchanger — 0.5–1.5 pH units higher than the protein’s pI of interest
Cation exchanger — 0.5–1.5 pH units lower than the protein’s pI of interest
Charged functional groups attached to resin beads in ion-exchange resins attract biomolecules of the opposite charge. Cation exchange resins have a negative charge, whereas anion exchange resins have a positive charge.
Resins can also be classified as “weak” or “strong” exchangers. These phrases do not refer to ion binding strength but rather the amount to which the ionization state of the functional groups fluctuates with pH.
Strong exchangers do not vary and remain completely charged across a wide pH range, making separation optimization easier than weak exchangers. The most popular ion-exchange chromatography resins are summarized in Table 1.
On the other hand, weak ion exchangers have a pH-dependent function and provide optimal performance over a narrow pH range. These resins suffer severe capacity loss when the pH of the buffer no longer matches the acid dissociation constant (pKa) of the resin functional group. Weak anion exchangers perform poorly at pH 9, and weak cation exchangers lose ionization below pH 6. When working with weak ion exchange resins such as diethylaminoethyl (DEAE) or carboxymethyl (CM), it is critical to stay within the operating pH range specified by the supplier.
Because their performance is unaffected by pH, strong ion exchangers have often selected resins for various applications. However, weak ion exchangers can be effective separation tools when strong ion exchangers fail because the selectivities of weak and strong ion exchangers frequently differ.
Particle size does not affect resin selectivity. However, it does affect resolution. Larger beads give resolutions suitable for early and intermediate phases of purification. Smaller beads offer the highest resolution and are appropriate for the latter stages of purification when purity is critical. This method enables target molecule binding and contaminant removal under various process conditions. At higher salt concentrations, bound molecules are freed from the matrix, allowing for separation based on the net charge of the molecules. Theoretically, a protein might bind to a cation or anion exchange resin. Still, proteins are only stable within a small pH range, and the protein’s stability determines the resin of choice at a certain pH.
The selection of the appropriate support matrix (stationary phase) is the foundation of the entire technique. The matrix used in chromatography media is typically a particle or resin packed into a column. The sample is placed into the column’s top and flushed with the mobile phase liquid, either by gravity or under pressure. There are hundreds of chromatography matrices in the market, such as cross-linked agarose, cellulose, dextran, polyacrylamide, latex, and controlled pore glass. Those chromatography mediums have many advantages but also often represent drawbacks such as larger particle size (>10um), less mechanical and chemical stability, and challenges to adopting automated workflows. Recently, there has been a surge in interest in developing and deploying magnetic separation techniques that use small magnetic particles.
Magnetic beads have significant advantages over non-magnetic bead technologies. They thus see increasing use in various areas of life-sciences research and development, including drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics. This is because magnetic beads have numerous advantages, including their ease of use, rapid experimental protocols, suitability and convenience for high-throughput automated and miniaturized processing, such as high-throughput screening, and the potential for scalability due to the availability of large homogenous biomagnetic separation equipment. Magnetic affinity and ion-exchange separations have been used successfully in various domains, including molecular biology, biochemistry, immunochemistry, enzymology, analytical chemistry, and environmental chemistry.
We offer strong cation exchange (SCX), strong anion exchange (SAX), weak cation exchange (WCX), and weak anion exchange (WAX) magnetic beads (Table 1). Our ion exchange (IEX) beads are rigid polymeric beads with covalent surface chemistries, which allow for easier handling and packing while providing greater physical and chemical stability—resulting in a robust production process. The beads are manufactured using nanometer-scale superparamagnetic iron oxide as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide. The pure inert silica makes less nonspecific binding. The beads are much smaller (1 and 5 µm diameter) in size and are non-porous, which exhibit larger surface area, less nonspecific binding, and higher resolution than porous supports. The beads enable instant and efficient capture of the target molecules. The physical and chemical properties of the beads will allow them for efficient high-throughput purification in parallel and make the best performance and highly reproducible results in both manual and automated instrument-based assay and separation systems.
Table 1 – Ion exchange magnetic beads
Beads
Feature
●
A Strong Anion Exchange (SAX) Beads, pKa =~12
●
Used to extract weaker cations that may not bind to WAX Magnetic Beads
●
A Weak Anion Exchange (WAX) Beads, pKa=~11.5
●
Used to extract very strong cations that may be retained irreversibly to SAX Magnetic Beads
●
A Weak Cation Exchange (WCX) Beads, pKa =~4.7
●
Used to extract very strong Anions that may be retained irreversibly to SCX Magnetic Beads
●
A Strong Cation Exchange (SCX) Beads, pKa =~2.3
●
Used to extract weaker Anions that may not bind to WCX Magnetic Beads
●
A Mixed-mode Ion Exchange Magnetic Beads
●
Hydroxyapatite has two adsorbing sites; a calcium site that binds with the acidic groups and a phosphate site that interacts with basic protein groups.
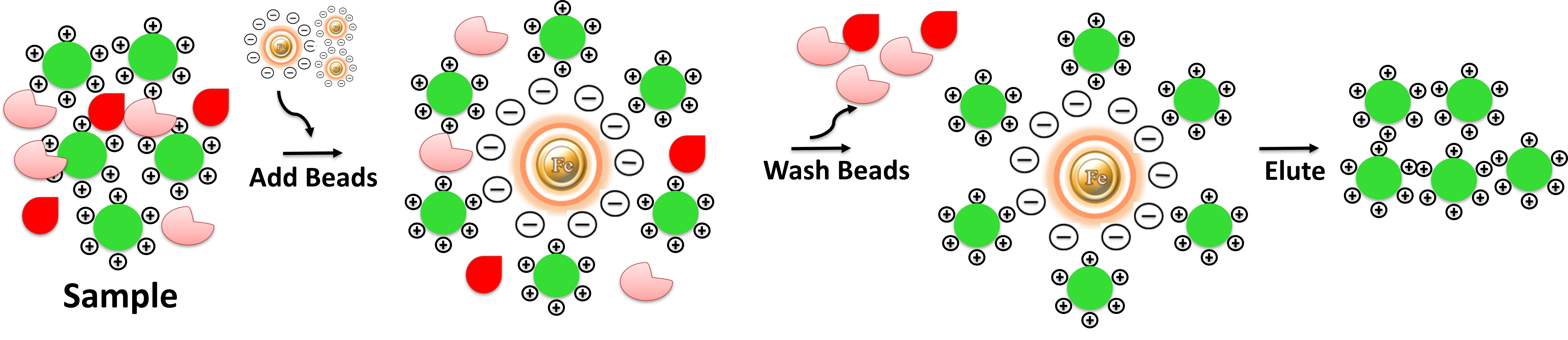
Ion Exchange purification is surprisingly easy. Compared with other Ion Exchange matrices, the beads avoid tedious centrifugation, precipitation, filtration, or columns. They enable easy washing, separation, and concentration of your target. The protocol involves three steps and takes less than 20 minutes of processing time. 1. Binding: Mix an impure sample with the beads in appropriate buffer conditions, vortex, or manual pipetting to capture the target molecules. 2. Washing: washed to remove other impurities. 3. Elution: Elute the target molecule using either a salt gradient or a change in pH. After loading an impure protein sample onto an ion-exchange chromatography column, the column is washed to remove unwanted proteins and other impurities. The protein(s) of interest are eluted using either a salt gradient or a pH change.
1.
Coskun O (2016) Separation techniques: Chromatography. North Clin Istanb 3:156–160
2.
Luqman, Mohammad, and Inamuddin (2012). Ion Exchange Technology II. Springer Netherlands. p. 1.
3.
Ninfa, Alexander; Ballou, David; Benore, Marilee (26 May 2009). Fundamental Laboratory Approaches for Biochemistry and Biotechnology. Wiley. pp. 143–145a
4.
Siegel, Miles (May 1997). “Rapid purification of small molecule libraries by ion exchange chromatography”. Tetrahedron Letters. 38 (19): 3357–3358
5.
Fritz, J. S. (2004). “Early milestones in the development of ion-exchange chromatography: a personal account”. Journal of Chromatography A. 1039 (1–2): 3–12.
6.
Eith, Claudia, Kolb Maximilian, and Seubert Andreas (2002). “Introduction” to Practical Ion Chromatography An Introduction. Ed. Viehweger Kai. Herisau: Metrohm. p. 160
7.
Ion Exchange Chromatography Principles and Methods. General Electric Company. 2004. pp. 11–20
8.
Jungbauer, Alois; Hahn, Rainer (2009). “Chapter 22 Ion-Exchange Chromatography”. Guide to Protein Purification, 2nd Edition. Methods in Enzymology. Vol. 463. pp. 349–371
Magnetic Beads Make Things Simple