- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
Specification
Composition
Magnetic Bead grafted with DVS group on the surface
Number of Beads
~ 1.68 x 109 beads/mg (1μm beads)
~ 5 x 107 beads /mg (5μm beads)
Stability
Short Term (<1 hour): pH 3-11; Long-Term: pH 4-10
Temperature: 4°C -140°C; Most organic solvents
Magnetization
~40-45 EMU/g
Type of Magnetization
Superparamagnetic
Formulation
Lyophilized Powder
Functional Group Density
1μm Magnetic Beads
~250 μmole / g of Beads
5μm Magnetic Beads
~200 μmole / g of Beads
Storage
Ship at room temperature. Store at 4°C upon receipt.
DVS-Activated Magnetic Beads are specially designed uniform beads that come pre-activated with high-density DVS functional groups on the surface. These beads can covalently conjugate ligands containing primary amino, sulfhydryl, or hydroxyl groups, making them a versatile option for a wide range of applications. The hydrophilic surface of the beads ensures low nonspecific adsorption and easy handling in various buffers. BcMag™ DVS-activated magnetic beads are particularly useful for immobilizing sugars and carbohydrates via their hydroxyl groups, and the long-arm hydrophilic linker reduces steric hindrance, making them an ideal affinity matrix for immobilizing large molecules or small peptides.
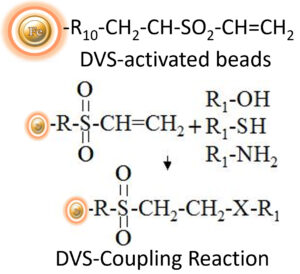
Protocol
Note:
●
The following protocol is an example for coupling protein and peptides to BcMag™ DVS -Activated magnetic beads. We strongly recommend performing a titration to optimize the concentration of beads used for each application. This protocol can be scaled up and down accordingly.
●
Coupling buffer containing an appropriate amount of PEG (PEG 20,000) may enhance coupling efficiency and coupling capacity, such as 5 – 7% PEG (w/v, final concentration) for antibody conjugations and 7-10% PEG (w/v, final concentration) for other proteins conjugation.
Materials Required
●
●
●
Block Buffer: 1 M ethanolamine, pH 9.0
●
30% PEG 20,000 solution
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
A.
Land Preparation
Note:
●
Coupling efficiencies to DVS-activated magnetic beads vary from ligand to ligand. The user should empirically optimize the concentration of the ligand. Recommend 0.5-10 mg/ml for protein or 200 μmoles ligands per ml for small peptide.
●
Avoid tris or other buffers containing primary amines or other nucleophiles because these will compete with the intended coupling reaction. But the wash or storage buffers can contain amino.
1.
Dissolve 0.5-10mg protein/peptide in 1ml coupling buffer containing appropriate PEG. If samples have already been suspended in other buffers, dilute samples with an equal volume of coupling buffer.
B.
Magnetic beads preparation
1.
Prepare 3% magnetic beads with 100% isopropanol (30 mg/ml).
Note: It has been stabling for over a year. Store the unused beads in isopropanol solution at 4°C.
2.
Transfer 100 μl (3mg) magnetic beads to a centrifuge tube.
3.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Remove the tube from the rack and resuspend the beads with 1 ml coupling buffer by vortex for 30 seconds.
4.
Repeat step 3 two times.
5.
Remove the supernatant, and the washed beads are ready for coupling.
Note: Once rehydrated, use the Bead as soon as possible due to the stability of the functional group.
C.
Coupling
1.
Add 100 μl of ligand solution to the washed magnetic beads and incubate overnight at room temperature with continuous rotation.
2.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Remove the tube from the rack and resuspend the beads with 1 ml wash buffer by vortex for 30 seconds. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Block the excess active groups on the beads by suspending the beads in 1ml Block buffer and incubate 30-60 minutes at room temperature with continuous rotation.
4.
Wash the beads with 1ml Wash buffer four times as described in C2.
5.
Resuspend the beads in PBS buffer containing 0.05% sodium azide and store them at 4°C.
D.
General Affinity Purification Protocol
Note:
●
This protocol is a general affinity purification procedure. Designing a universal protocol for all protein purification is impossible because no two proteins are precisely alike. The user should determine the optimal working conditions for purifying the individual target protein to obtain the best results.
●
We strongly recommended titration to optimize the number of beads used for each application based on the amount of the target protein in the crude sample. Too many magnetic beads used will cause higher backgrounds, while too few beads used will cause lower yields. Each mg of magnetic beads typically binds to 10-20 μg of the target protein.
1.
Transfer the optimal amount of the beads to a centrifuge tube. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
2.
Remove the tube and wash the beads with 5-bed volumes of PBS buffer by vortex for 30 seconds. Leave the tube at room temperature for 1-3 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Repeat step 2 two times.
4.
Add washed beads to the crude sample containing the target protein and incubate at room or desired temperature for 1-2 hours (Lower temperatures require longer incubation time).
Note: Strongly recommended to perform a titration to optimize incubation time. More prolonged incubation may cause higher background.
5.
Note: Adding a higher concentration of salts, nonionic detergent, and reducing reagent may reduce the nonspecific background. For example, adding NaCl (up to 1-1.5 M), 0.1-0.5% nonionic detergents such as Triton X 100 or Tween 20, and a reducing reagent such as DTT or TCEP (we usually use 3mM) to the washing buffer.
6.
Elute the target protein by appropriate methods such as low pH (2-4), high pH (10-12), high salt, high temperature, affinity elution, or boiling in an SDS-PAGE sample buffer.
Note: The linkage chemistry of DVS coupled ligand is unstable in alkaline conditions; acid elution is recommended when the pH elution method is desired.
Magnetic Beads Make Things Simple