- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Fluorescent probes are molecules that absorb light of one wavelength and emit light of another, usually a longer wavelength (a process known as fluorescence). Fluorescent molecules are widely used in biological research, and their popularity is growing due to their variety, sensitivity, and quantitative capabilities. Fluorescent probes are used to detect protein localization and activation, identify protein complex formation and conformational changes, and monitor biological processes in vivo, among other things. This representative image was created using immunofluorescence techniques.
Fluorescent compounds, commonly known as fluorophores or simply fluors, respond differently to light than other molecules. A photon of excitation light is absorbed by an electron of a fluorescent particle, raising the electron’s energy level to an excited state. During this brief excitation phase, some energy is wasted by molecular collisions or transferred to a nearby molecule, and the remainder is emitted as a photon to return the electron to its ground state. Because the released photon typically carries less energy and has a longer wavelength than the excitation photon, the emitted fluorescence can be separated from the excitation light. A fluorophore’s excitation and photon emission are cyclical, and it can be excited repeatedly until irreparably damaged. Fluorophores can thus emit multiple photons through this cycle of Excitation and emission, and fluorescent molecules are consequently used for a wide range of research purposes.
Fluorescence has been employed in biological study for over a century, but advances in fluorescence chemistry, as well as technological breakthroughs, have fuelled the development of a wide range of fluorophores. Today’s broad array of fluorophores offers greater flexibility, variation, and fluorophore performance than ever before for research purposes. Fluorophores are classified into three broad categories:
Organic Fluorescent Dyes
Fluorescent dyes, also known as fluorophores/reactive dyes, are non-protein molecules typically containing numerous aromatic groups or plane or cyclic moieties. Fluorescent dyes are widely used in life science due to their higher photostability, sensitivity, and selectivity in target detection when compared to fluorescent proteins; however, they still suffer from the limitations of small Stokes shifts, insufficient fluorescent efficiency, low photostability, and autofluorescence interference for precise quantification.
Fluorescent Proteins
Fluorescent proteins are genetically encoded protein sequences containing fluorescent amino acids (tryptophan, tyrosine, and phenylalanine), which absorb excitation light and then reemit emission light. Fluorescent proteins are widely used as Biomarkers and Biosensors for monitoring protein-to-protein interaction, visualizing protein localization, and detecting transgenic expression in vivo.
Quantum Dots
Quantum dots are nanometer-sized semiconducting materials with diameters of 2 to 50 nm. Quantum dots, with their unique optical properties, emit light of specific wavelengths based on their shape, material composition, and size if energy is applied to them. Quantum dots are widely used for immunolabeling, multiplexed biological detection, and molecular imaging, both in vitro and in vivo assays. One of the main disadvantages of quantum dots is their intermittent luminescence (blinking), which may interfere with some molecular identification methods.
Although those conventional fluorophores have been widely used over the past decades, they are still suffering from either one or several limitations in terms of applicability and efficiency:
1.
Narrow excitation bands cause higher background signals.
2.
Smaller Stokes shift often produces self-quenching.
3.
Fluorescence is sensitive to environmental factors such as metallic ion concentration, pH, temperature, and solvent polarity.
4.
Fluorescence intensity is not high enough for detecting a single biomolecular.
5.
Fluorescence intermittency (blinking) affects some processes of molecule detection.
6.
Only a few bioconjugatable forms are commercially available.
7.
It is easily aggregated because of hydrophobicity.
More improved fluorescence techniques have been developed to address the shortcomings of today’s fluorophores. TR-FRET is one of them, and it has proven to be a highly versatile assay technique, allowing researchers to explore a broad spectrum of biological interactions with low to high affinity, employing both small and large compounds.
Förster resonance energy transfer (FRET) is based on the transfer of energy between two fluorophores in close proximity, a donor (long-lived fluorescence) and an acceptor (short-lived fluorescence) (Fig.1). The level of energy transfer between biomolecules can be measured by labeling each partner with a fluorescent marker and measuring the level of interaction. Organic luminous chemicals such as fluorescein and rhodamine were once commonly utilized in fluorescence assays. However, these bioassays have significant disadvantages in that fluorescent detection is significantly inhibited by noise in the background derived from scattered excitation light and interfered with by fluorescence from coexisting material in the sample (fluorescent compounds and dust/line), making obtaining a highly sensitive measurement difficult.
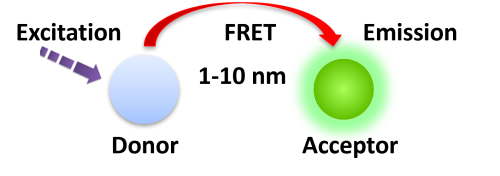
BcMag™ TR-FRET Assay, in contrast to typical FRET assays, uses time-resolved fluorescent magnetic beads (BcMag™ TR-Magnetic Beads) as the donor fluorophore. The donor and acceptor can be two proteins, two DNA strands, an antigen, an antibody, or a ligand and its receptor. After a reasonable time delay (usually 50 to 100 s), a signal is generated by fluorescence resonance energy transfer between a donor and an acceptor molecule when they are close and monitored in a time-resolved way. In BcMag™ TR-FRET Assay, a trace amount of analytes can be easily enriched from the complex by TR-Magnetic Beads, resulting in higher sensitivity. This assay practically eliminates all fluorescence backgrounds caused by the sample and plastic microplate, as well as by direct acceptor excitation. As a result, the signal-to-noise ratios of the BcMag™ TR-FRET Assay are very high, and the background is quite low. Furthermore, the assay does not need washing steps. BcMag™ TR-FRET Assay offers substantial advantages to bioassays in high throughput screening, such as assay flexibility, dependability, increased assay sensitivity, higher throughput, and fewer false positive/false negative results.
Magnetic bead separation is a fast, effective, and clean method used by scientists to replace filtering, centrifugation, and separation processes. Time-resolved Fluorescence Magnetic Beads can be used for immunoassays and other applications. They have high surface-to-volume ratios, small sizes (0.1-10µm), various functional groups attached to the surfaces (e.g., antibodies, DNA, and chemical groups), and the ability to manipulate the particles via an applied magnetic field easily. Combined with automated liquid handling and robust detection instrumentation, these characteristics enable a wide range of high-throughput applications.
Table 1. Fluorescence Properties of TRF Magnetic Beads
Fluorophore
Red
Green
Far-Red
340
320
470
615
545
710
Fluorescence lifetime (Ԏ) (µsec)
730
1050
354.36
Stokes shifts
(nm)
275
220
175
Fluorophore
Fluorescence lifetime (Ԏ) (µsec)
Stokes shifts
(nm)
Red
340
615
730
275
Green
320
545
1050
220
Far-Red
470
710
354.36
175
1.
Perform a double function simultaneously on the same beads: The magnetic beads combine separation/preconcentration and detect analytes, allowing quick, simple, robust, and high-throughput analytes of trace amounts from complex biological samples on the same beads.
2.
Ultra sensitive. Lower detection limits of 10 pg/mL versus typical fluorometric detection limits of 100 pg/mL.
3.
Extremely photostable and highly resistant to photobleaching. All the lanthanides chelate or cryptate molecules and iron oxide are entirely encapsulated inside each bead instead of merely on the bead’s surface. The protective environment prevents iron oxide and dye from leaching into aqueous media, which makes the beads less sensitive to external conditions such as solvent, temperature, pH, etc.
4.
Very high fluorescent intensity. Because a single bead has a large concentration of lanthanide chelate with a high quantum yield ranging from 40 to 90%, the beads show excellent fluorescence intensity, which increases test sensitivity without signal amplification. Such bright beads are also perfect for donors’ use in time-resolved FRET assays.
5.
Lanthanides chelate or cryptate has large Stokes shifts (>250 nm), narrow emission bands (-10 nm bandwidth), and long fluorescence lifetime (μs), which dramatically reduces background and increases the signal-to-noise ratio.
6.
Most bioprocess ELISA assays can be converted to an HTRF assay.
7.
No washing step is involved in the assays.
8.
Have a hydrophilic silica surface grafted by different functional groups with linkers of variable lengths, allowing efficient conjugation of various ligands such as peptides, proteins, antibodies, small molecules, carbohydrates, aptamers, DNA/RNA, etc.
9.
Due to the microsphere’s magnetic properties, the fluorescent magnetic beads are suitable for high-throughput automation.
The TRF beads assay is straightforward.
1.
Mix the antibody-conjugated donor beads with the cell lysates and incubate them with continuous rotation for a sufficient time. The beads remain suspended in the sample solution during mixing, allowing the target analytes to bind to the donor beads.
2.
After incubation, the beads are collected and separated from the sample using a magnet rack.
3.
Add the antibody-conjugated acceptor and incubate them with continuous rotation for a sufficient time.
4.
Analysis of numerous microplate readers supports TR-FRET measurements.
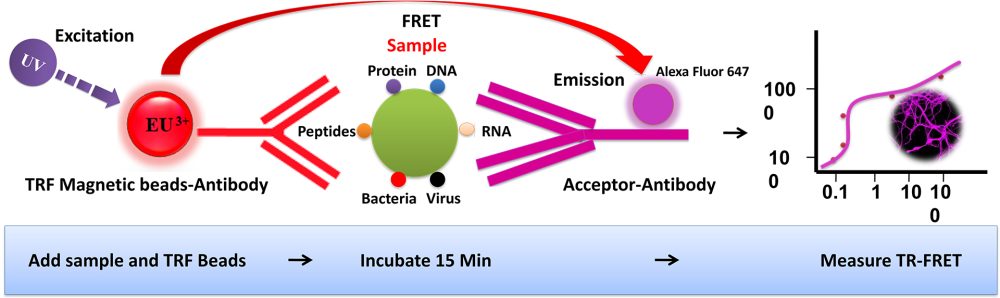
Table 2. Selection of TRF Magnetic Beads and Acceptor
TRF-Magnetic Beads
Excitation
Emission
Acceptor
BcMag™ Europium Fluorescent Magnetic Beads
340 nm
615 nm
BcMag™ Europium Fluorescent Magnetic Beads
340 nm
615 nm
AlexaFluor 647
BcMag™ Europium Fluorescent Magnetic Beads
340 nm
615 nm
XL665/d2
BcMag™ Terbium Fluorescent Magnetic Beads
320 nm
545 nm
Fluorescein / GFP
BcMag™ Ruthenium Fluorescent Magnetic Beads
470 nm
710 nm
Far-red Dye
Fluorescence Detection and measurement can be used using the following instruments:
1.
Molecular Devices, LLC.
●
●
2.
Thermo Fisher Scientific
●
Varioskan LUX Multimode Microplate Reader
3.
Medisensor, Inc.
●
Qcare™ TRF-S reader
4.
BioTek
●
Cytation 1 Cell Imaging Multimode Reader
5.
●
EnVision 2104-0020 Multilabel Microplate Reader Pred 2105
●
EnSpire 2300 MultiMode Microplate Reader
6.
BMG LABTECH
●
1.
Corr SA, Rakovich YP, Gun’ko YK. Multifunctional Magnetic-fluorescent Nanocomposites for Biomedical Applications. Nanoscale Res Lett. 2008 Mar 6;3(3):87–104.
2.
Diamandis EP 1988. Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem. 1988 Jun;21(3):139-50.
3.
Fan W, Liu D, Ren W, Liu C. Trends of Bead Counting-Based Technologies Toward the Detection of Disease-Related Biomarkers. Front Chem. 2020 Dec 21;8:600317.
4.
Leng, Y., Sun, K., Chen, X., and Li, W. (2015). Suspension arrays based on nanoparticle-encoded microspheres for high-throughput multiplexed detection. Chem. Soc. Rev. 44, 5552–5595
5.
Ma, F., Li, Y., Tang, B., and Zhang, C. (2016). Fluorescent biosensors based on single-molecule counting. Acc. Chem. Res. 49, 1722–1730
6.
Parsa, S. F., Vafajoo, A., Rostami, A., Salarian, R., Rabiee, M., Rabiee, N., et al. (2018). Early diagnosis of disease using microbead array technology: a review. Anal. Chim. Acta 1032, 1–17.
7.
Werts, M. H. V. (2005). “Making Sense of Lanthanide Luminescence.” Science Progress. 88 (2): 101–131
8.
Rödiger, S., Liebsch, C., Schmidt, C. et al. Nucleic acid detection based on the use of microbeads: a review. Microchim Acta 181, 1151–1168 (2014).
9.
Soini, Erkki; Lövgren, Timo; Reimer, Charles B. (January 1987). “Time-Resolved Fluorescence of Lanthanide Probes and Applications in Biotechnology”. C R C Critical Reviews in Analytical Chemistry. 18 (2): 105–15
10.
Satoshi Sakamoto, Kenshi Omagari, Yoshinori Kita, Yusuke Mochizuki, Yasuyuki Naito, Shintaro Kawata, Sachiko Matsuda, Osamu Itano, Hiromitsu Jinno, Hiroya Takeuchi, Yuki Yamaguchi, Yuko Kitagawa, Hiroshi Handa, Magnetically Promoted Rapid Immunoreactions Using Functionalized Fluorescent Magnetic Beads: A Proof of Principle, Clinical Chemistry, Volume 60, Issue 4, 1 April 2014, Pages 610–620
Magnetic Beads Make Things Simple