- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Polyclonal antibodies (serum samples) are a mixture of unrelated IgG and antigen-specific IgG. The antigen-specific IgG generally accounts for only 2-5% of total IgG in serum. The antigen-specific IgG is often required to quantify the relative amount of the antigen-specific antibody for applications such as ELISA, neutralization, immunohistochemistry, and Western blotting since large quantities of the unrelated antibody can increase background noise. Proteins A, G, A/G, or L are excellent tools for purifying total IgG from various samples, but with the problem of co-purification of nonspecific immunoglobulins. Therefore, it cannot purify antigen-specific antibodies from the antiserum. Isolation of these antibodies by other purification methods (e.g., ion-exchange chromatography) is tedious and ineffective. On the other hand, immunoaffinity purification, which exploits the specificity of antibody-antigen interactions, provides many advantages such as very high purity levels (>95% can be achieved by using a specific 3-step procedure), very high selectivity, and therefore very high resolution. Antigen-specific affinity purification of antibodies involves antigen immobilization to a solid support and antibody purification from the serum.
Any molecule that induces the immune system to develop antibodies against it is referred to as an antigen. Antigens are typically proteins or polysaccharides with a large molecular weight. Antigens can also be polypeptides, lipids, nucleic acids, and various other substances. Immune responses can be elicited against smaller molecules known as haptens when they are chemically attached to a bigger carrier protein such as bovine serum albumin, keyhole limpet hemocyanin (KLH), or other synthetic matrices. Haptens can be compounds such as medicines, simple sugars, amino acids, short peptides, phospholipids, or triglycerides. As a result, given enough time, almost any foreign material will be recognized by the immune system and elicit particular antibody formation. However, this unique immune response is highly varied and is heavily influenced by antigen size, shape, and composition. Proteins or glycoproteins are thought to be the best antigens because of their potential to elicit a potent immune response; in other words, they are highly immunogenic.
Making antigen-specific affinity supports requires methods to covalently conjugate antigens to an appropriate insoluble support (matrix). Antigen-specific affinity purification of antibodies involves two processes: antigen immobilization to a solid support and antibody purification procedure from the serum. Immobilization and conjugation refer to the covalent attachment of an antigen to a solid support through their common chemical groups. This method prepares the matrices by first activating with reactive chemical compounds toward one or more of these functional groups. These chemical groups should be easily activated, such as primary amines, sulfhydryls, aldehydes, carboxylic acids, etc. The activated matrices then can conjugate antigens through a covalent linkage, resulting in ligand immobilization.
1.
Choice of solid supports
Although antigen-specific affinity purification of antibodies is an efficient method, most support matrices consist of the agarose resin or its derived column. The problems with their procedures are tedious, time-consuming, unable to handle large samples volume in a short time, and challenging to adapt to the automation system. New affinity resins with higher efficiency, homogeneity, and high throughput are required. Affinity ligands are now coupled to magnetic particles that facilitate more efficient capture of specific biomolecules.
Magnetic beads (particles) are an entirely different type of solid support matrices from beaded agarose or other porous resins. They are much smaller (typically 1-5 µm diameter), thus providing larger surface areas for a high density of ligand immobilization. The beads are manufactured using nanometer-scale superparamagnetic iron oxide as core and entirely encapsulated by a high purity silica shell, ensuring no leaching problems with the iron oxide. The excellent hydrophilic properties of the beads make them less nonspecific binding. Bioclone offers many activated functional groups and variable-length hydrophilic linkers for conjugating almost all the antigens. Additionally, the beads combine all the advantages of antigen-specific antibody purification (low costs, simplicity, high specificity, and capacity) and magnetic properties to perform efficient manual or automatic quick high-throughput purification.
2.
Immobilization chemistry
The successful antibody or antigen-specific affinity purification of antigen or antibodies depends on the availability and effectiveness of the relevant epitope on the antigen to the binding site of the antibody and immobilization efficiency of the antigen to the affinity support matrix. The following general considerations apply when antigens are immobilized to solid affinity supports.
a.
Linkers/spacers: Linkers are flexible molecules or stretch of molecules that link two molecules of interest together. The linkers’ property affects the affinity support’s performance in several ways.
●
Linker length. Suppose ligands are small peptide antigens or molecular. In that case, it is best to use a long arm linker (>10 atoms) attached to the support matrix since it can avoid the steric hindrance (The ligand is inaccessible to the binding site). Linker length is less critical for large (e.g., protein) antigens since the antigen itself is an effective linker between the support matrix and the epitope.
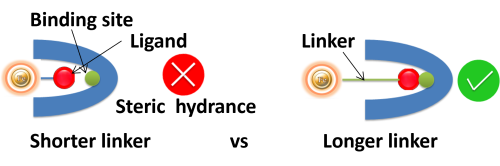
●
Site-directed immobilization: Using a unique functional group for site-directed immobilization is beneficial for correct orientation, such as sulfhydryl on a single terminal cysteine in a peptide for a small antigen) and carbohydrate residues on the Fc side for antibody.
●
Use hydrophilic linker to immobilize antigen to activated support to reduce background noise.
●
Use a cleavable linker to separate the target from the solid matrix easily.
b.
Reaction groups
The antigen is crosslinked with some carrier protein for the small antigen to make it immunogenic. For best results, we recommend that the antigen is immobilized to affinity purification using the same chemistry (e.g., reaction to primary amines, sulfhydryls, carboxylic acids, or aldehydes) for crosslinking the small antigen to the carrier protein. This way allows all epitopes to become available for antibody binding. Figure below summarizes the selection of chromatography resin selection for immobilizing antigens.
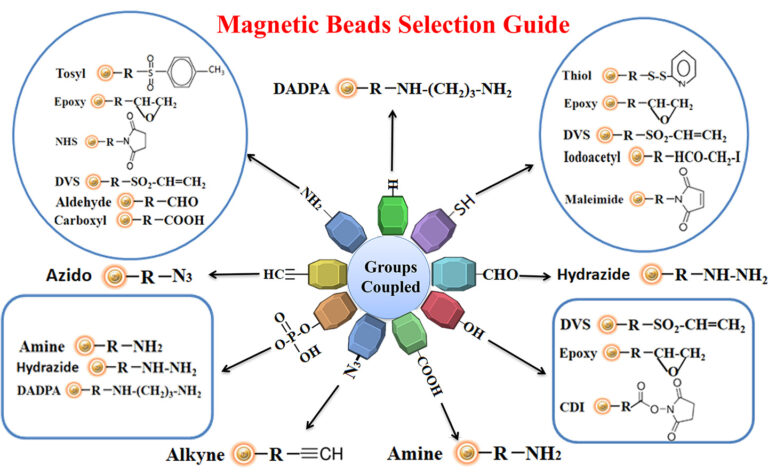
Learn More
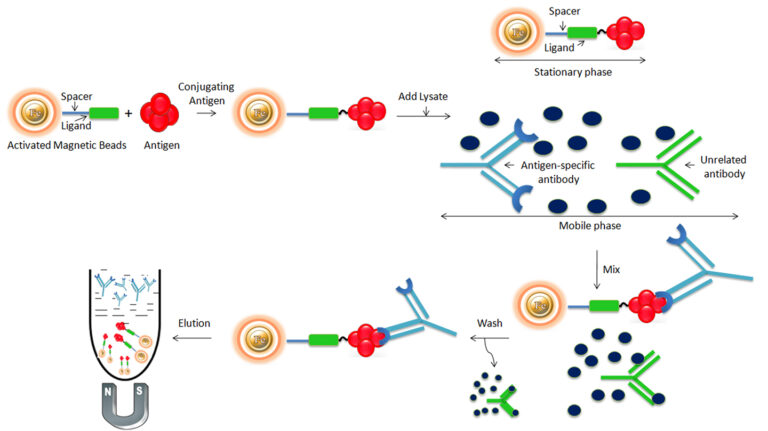
Once the antigen is immobilized to the solid support, it is ready for antibody purification. The purification with magnetic beads is straightforward. Affinity purification with magnetic particles is not performed in-column; instead, by simple bench-top procedures.
1.
Bind: Mix a clarified, physiologic-buffered (pH 7 to 8) sample of the antiserum with the magnetic beads to allow the antibody to bind to the immobilized antigen. The binding buffer used with the affinity beads should have an appropriate pH and ionic strength to promote binding between the immobilized antigen and antibody.
2.
Wash: Wash the beads with phosphate-buffered saline (PBS) by the magnetic rack to wash away nonspecific proteins. Use a volume of wash buffer 5 to 10 times the bead volume.
3.
Elute: Elute the antibody from the beads by adding elution buffer (e.g., 0.1M glycine-HCl, pH 2.8). The low-pH condition dissociates the antibody from the immobilized antigen, and the antibody is recovered in its purified state in one or several elution fractions. The Eluted antibody should be immediately neutralized by adding 1/10th volume of 1M Tris-HCl (pH 8.5) to protect the antibody.
Feature and benefits
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Stable covalent bond with minimal ligand leakage
●
High binding capacity, very low nonspecific binding
●
Scalable – easily adjusts for sample size and automation
●
Reproducible results
1.
Sheng S, Kong F. Separation of antigens and antibodies by immunoaffinity chromatography. Pharm Biol. 2012 Aug;50(8):1038-44. doi: 10.3109/13880209.2011.653493. Epub 2012 Apr 6. PMID: 22480305.
2.
Ma H, O’Kennedy R. The Purification of Natural and Recombinant Peptide Antibodies by Affinity Chromatographic Strategies. Methods Mol Biol. 2015;1348:153-65. doi: 10.1007/978-1-4939-2999-3_15. PMID: 26424271.
3.
Darcy E, Leonard P, Fitzgerald J, Danaher M, O’Kennedy R. Purification of antibodies using affinity chromatography. Methods Mol Biol. 2011;681:369-82. doi: 10.1007/978-1-60761-913-0_20. PMID: 20978976.
4.
Ayyar BV, Arora S, Murphy C, O’Kennedy R. Affinity chromatography as a tool for antibody purification. Methods. 2012 Feb;56(2):116-29. doi: 10.1016/j.ymeth.2011.10.007. Epub 2011 Oct 19. PMID: 22033471.
5.
Arora S, Ayyar BV, O’Kennedy R. Affinity chromatography for antibody purification. Methods Mol Biol. 2014;1129:497-516. doi: 10.1007/978-1-62703-977-2_35. PMID: 24648096.
Magnetic Beads Make Things Simple