- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

A viral disease (also known as a viral infection, abbreviated vid or VID) arises when pathogenic viruses invade an organism’s body and infectious virus particles (virions) bind to and penetrate vulnerable cells. Viral diseases are widespread and cause a wide range of viral disorders. A virus is made up of nucleic acid, either DNA or RNA, encased in a protein coat. Sometimes, a lipid envelope the protein coat when it is outside of a cell. Virus shapes range from simple helical and icosahedral structures to more complicated configurations. The average virus is about one-hundredth the size of a bacterium and cannot be seen with a standard light microscope. Since the virus is acellular, it cannot complete the development process independently. They must copy and assemble themselves using the host cell. A virus’s life cycle comprises six stages: attachment, incursion, synthesis, shell, and release. The viral capsid protein first recognizes and binds the particular receptor on the host cell surface. Second, following attachment, the virus enters the cell by a membrane or cell receptor-mediated fusion. Then, finish the viral genome replication, transcription, and protein synthesis. Finally, the viral copies are released by the host cells.
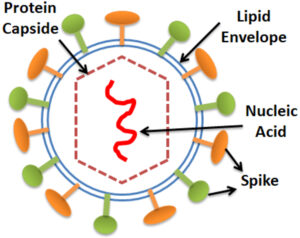
An antigen is anything that stimulates an immunological reaction in another organism. This immune response might be as simple as an increase in inflammatory markers or as complex as activation of the adaptive immune system and antibody production. Antibodies include two or more distinct paratopes, or antigen recognition sites, that allow them to recognize and battle the invading antigen. The number of antigen recognition sites varies according to antibody class. Any protein of interest detected by a bioassay or bio detection platform can also be referred to as an “antigen”.
A viral antigen is a multi-antigenic protein antigen that is strain-specific and intimately connected with the virus particle. It is found on the surface of viruses that trigger an immunological response known as antibody formation in its host. Viral antigens protrude from the capsid and frequently play essential roles in docking to the host cell, fusion, and viral DNA/RNA injection. Antibody-based immune responses provide the host with the initial line of defense against viral infection; nevertheless, in many circumstances, a robust cellular immunological response mediated by T-cells and NK-cells is necessary for efficient viral clearance. When cellular immunity fails to clear the virus, the infection can become chronic, and serum antibodies to the viral pathogen are utilized to make the diagnosis.
Immunodetection is a valuable technique for detecting and diagnosing viral infection. Even when a virus is unculturable, it is possible to respond quickly to emerging viruses and novel viral strains of known diseases if the gene sequence is available. The ability to synthesize recombinant viral proteins will ensure that future Immunodetection is safe, specific, and fast. Recombinant viral antigens contain a portion of the viral sequence, which means that the recombinant antigen has a region that antibodies generated by different people can identify. It decreases the possibility of false negatives, which can occur with synthetic peptides because they only contain a small piece of the whole protein. A false negative result occurs if an individual infected with a viral antigen develops antibodies against a portion of the protein that is not included in the synthesized peptides.
A fusion protein is typically included in recombinant viral protein, resulting in improved adhesion to test surfaces such as wells. As a result, lesser quantities of recombinant protein will generate the same results as greater quantities of unfused protein. The selection of a fusion partner eliminates false positives, allowing for superior adhesion without inaccurate results. Bacteria, yeast, mammalian cells, and viruses can produce recombinant viral antigens. Although produced antigens from E. Coli were not glycosylated, it proved in many cases that the non-glycosylated protein was just as immunogenic. The apparent advantage of recombinant viral antigens is their indefinite availability and ease of manufacture and quality control.
1.
Production and quality control are straightforward.
2.
There are no nucleic acids or any viral or foreign proteins; hence it is less hazardous.
3.
More secure when viruses are oncogenic or cause prolonged infection.
4.
Possibility, even if the virus cannot be cultured
1.
It May be less immunogenic than traditional inactivated whole-virus vaccinations.
2.
Adjuvant is required.
1.
Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Domen J, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MMG, McInnes MDF, Spijker R, Van den Bruel A. Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No.: CD013705. DOI: 10.1002/14651858.CD013705.pub2. Accessed 15 June 2022.
2.
Grandien M. Viral diagnosis by antigen detection techniques. Clin Diagn Virol. 1996 May;5(2-3):81-90. doi: 10.1016/0928-0197(96)00209-7. PMID: 15566866.
3.
Wirgart BZ, Claesson K, Eriksson BM, Brundin M, Tufveson G, Tötterman T, Grillner L. Cytomegalovirus (CMV) DNA amplification from plasma compared with CMV pp65 antigen (ppUL83) detection in leukocytes for early diagnosis of symptomatic CMV infection in kidney transplant patients. Clin Diagn Virol. 1996 Nov;7(2):99-110. doi: 10.1016/s0928-0197(96)00258-9. PMID: 9137866.
Magnetic Beads Make Things Simple