- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Components
Shipping conditions: At ambient temperature
Components
BcMag™ U-DNA Beads
5x Lysis Buffer E
10x Lysis Buffer P
Proteinase K
DTT
Proteinase K Suspension Buffer
Storage
4°C
4°C
4°C
-20°C
-20°C
4°C
Cat. No. AT101 (50 Preps)
1.5 ml
4.0 ml
250 µL
30 mg
7.7 mg
1.5 ml
Cat. No. AT102 (100 Preps)
3.0 ml
8.0 ml
0.5 ml
60 mg
15.4 mg
3.0 ml
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit components according to the table Above on arrival.
The BcMag™ Sexual Assault Casework DNA Purification Kit is the ultimate solution for quick and efficient purification of male DNA from trace amounts of sexual assault samples. Our kit uses novel negative selection chromatography magnetic beads that rapidly capture impurities, such as PCR inhibitors, from cell lysates, while leaving male DNA untouched. With this kit, the user can obtain pure male DNA with ease and confidence, knowing that the highest standards of purity and integrity are being met.
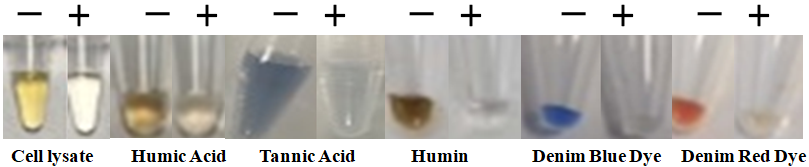
Reduce the risk of DNA loss and buffer carryover with the BcMag™ Sexual Assault Casework DNA Purification Kit. This kit eliminates the need for the time-consuming bind-wash-elute technique and provides a quick and easy way to purify DNA, while maintaining the highest standards of purity and integrity. With pure DNA obtained from this kit, quantitative PCR and STR analysis work seamlessly, providing you with accurate and reliable results.
●
Remove >95% epithelial cell DNA.
●
High purity and recovery rate of sperm DNA from a variety of trace samples
●
Rapid and efficient purification protocol: One tube, without prior sperm DNA isolation for subsequent use in direct workflows, and no liquid transfer.
●
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High throughput: Compatible with many different automated liquid handling systems
1.
Add Lysis Buffer to break the epithelial cells and release their DNA.
2.
Centrifuge to remove all epithelial cell DNA.
3.
Add Lysis Buffer P to lyse the sperm cells to release sperm DNA.
4.
Mix the lysate with the magnetic beads to capture the PCR inhibitors.
5.
Remove the beads with a magnet and aspirate the supernatant containing only the pure, ready-to-use sperm DNA.
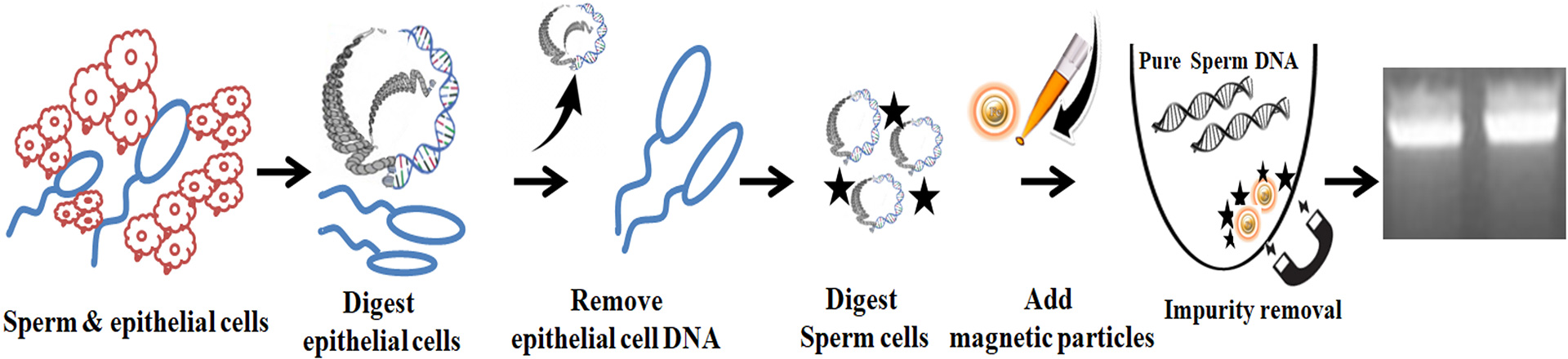
The following protocol is an example. The protocol can be scaled up or down as needed.
Notes
●
DNA Yield: Varies (depends on sample size and type)
●
DNA Size: Varies (depends on the quality of starting material)
●
Since there is no concentration step in the protocol, the concentration of the nucleic acid depends on the quality and quantity of the sample used.
●
Quantification of the nucleic acids: Use only fluorescence methods such as qPCR, Qubit, and Pico Green.
●
OD260 methods such as Nanodrop and UV-spectrophotometry are not-suitable.
●
For long-term storage, store the extracted nucleic acids at -20°C.
Materials Required by the User
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-06)
Item
Adjustable Single and Multichannel pipettes
Item
Centrifuge with swinging bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-06)
Adjustable Single and Multichannel pipettes
Centrifuge with swinging bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR Plates/Tubes
! IMPORTANT ! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
A. Sample pretreatment to remove the epithelial DNA
Sample Input
Sample
Example Sample Input
Seminal / Vaginal fluid mixture on fabric
Seminal / Vaginal fluid mixture on the swab
Up to one swab
! IMPORTANT !
1.
Proteinase K preparation: Provide protease K as lyophilized powder and dissolve at a 20 mg/ml concentration in Proteinase K Suspension Buffer. For example, 12.5 mg dissolved in 625 µl of Proteinase K Suspension Buffer. Divide the stock solution into small aliquots and store at -20°C. Each aliquot can be thawed and refrozen several times but should then be discarded.
2.
DTT solution preparation: Provide DTT as powder and dissolve at a concentration of 1M in ultrapure water. For example, 15.4 mg dissolved in 100µl ultrapure water. It is stable for years at -20°C. Prepare in small aliquots, thaw it on ice, and use and discard. Store them in the dark (wrapped in aluminum foil) at -20°C. Do not autoclave DTT or solutions containing it. Avoid multiple freeze-thaw cycles.
3.
Dilute DTT to a concentration of 10 mM from stock with ultrapure water and use it immediately. Discard unused DTT solution.
B. Procedure
1.
Bring the thermal shaker temperature to 65°C.
2.
Add the sample to a 1.5 ml centrifuge tube.
3.
To the tube that contains the sample, add 400 μLof lysis buffer E and 10 μl of Proteinase K.
Note: If multiple samples will be processed, scale up the volume of reagents used and prepare a master Lysis mix.
4.
Mix the sample by Vortex or invert the tube for 10-15 seconds.
5.
Place the tube in a thermal shaker (or water bath), then incubate it at 65°C for 1 hour.
6.
Use disposable tweezers to roll the sample against the tube sides, press the sample against the side to squeeze as much of the liquid as possible, and remove the sample.
7.
Centrifuge at room temperature and 14,000 rpm for 5 minutes.
8.
Remove the supernatant and save the sperm pellet.
9.
Repeat steps B3 to B8 once to completely remove the epithelial DNA.
10.
Proceed to Purification of sperm DNA, next section.
C. Purification of sperm DNA
Procedure
Premix Beads Solution Preparation
! IMPORTANT !
●
Before pipetting, shake or Vortex the bottle to completely resuspend the Magnetic Beads.
●
Do not allow the magnetic beads to sit for more than 2 minutes before dispensing.
1.
Prepare a fresh Master Mix following Table 2 for the number of samples to be processed, plus 10% more (e.g., if you have 10 samples, prepare Master Mix for 11). Add the following components to the reservoir.
Table 2. Premix Beads solution
1 Well / Tube
( 50 μL Reaction Volume)
BcMag™ U-DNA Beads
10x Lysis Buffer
30 μL
5 μL
Proteinase K (20mg/ml)
6.25 μL
DTT (10 mM)
Ultrapure Water
Total
1.5 μL
7.25 μL
50 μL
2.
To the PCR plate/tube that contains the sperm pellet, add 50 μL Premix Beads solution.
3.
Mix the sample by Vortex or invert the tube for 10-15 seconds.
4.
Place the PCR plate/tube into a thermocycler and incubate at:
a.
65°C for 1 hour
b.
80°C for 10 minutes
5.
Remove the PCR plate/tube from the thermocycler and mix the sample with beads by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 5 minutes.
6.
Centrifuge at 3500 rpm for 5 minutes.
7.
Place the sample plate/tube on the magnetic separation plate for 30 seconds or until the solution is clear.
8.
Transfer the supernatant to a clean plate /tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications. Using 1-5 μl for downstream applications.
D. Troubleshooting
Problem
Low DNA/RNA Recovery
Probable Cause
Poor starting sample material
Suggestion
Problem
Ct Value Delays
Probable Cause
Too many PCR inhibitors in the sample.
Suggestion
Problem
Ct Value Delays
Probable Cause
Recovery DNA is so low.
Suggestion
Problem
Probable Cause
Suggestion
Low DNA/RNA Recovery
Poor starting sample material
Ct Value Delays
Too many PCR inhibitors in the sample.
Recovery DNA is so low.
Magnetic Beads Make Things Simple