- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
The BcMag™ One-Step NGS Cleanup Kit is a cutting-edge solution that has been developed to eliminate the complexities involved in purifying DNA after adaptor ligation and PCR. This kit is a game-changer and can even replace the need for size selection post adaptor addition. The protocol is incredibly straightforward and requires only one tube and one step. The kit offers a high degree of flexibility, allowing for the removal of various size DNA fragments by merely adjusting the processing time, buffer pH, and detergent concentration.
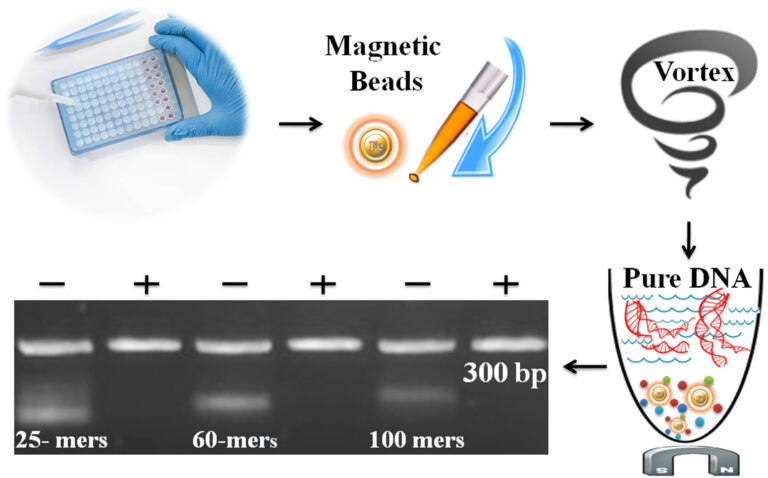
The kit’s magnetic beads are the real heroes here, as they are directly added to finished PCR reactions or other DNA reactions and are mixed by either a vortex mixer or pipetting to capture and remove impurities. The beads are capable of removing excess primer, dimer, adapter, salt, detergent, dNTPs, and enzyme, making them a valuable tool in any lab’s arsenal. After the mixing process, the beads are magnetically removed, while the purified DNA is ready for downstream applications in just one minute, which is a phenomenal time saver. These downstream applications include Sanger Sequencing, Restriction Digestion, Cloning, SNP Detection, or Library Preparation for NGS, making this kit incredibly versatile. Furthermore, the beads allow for the processing of 96 samples simultaneously in less than 10 minutes, making it a powerful and efficient tool.
●
Simple protocol: No liquid transfer, One-tube, One-step
●
Ultrafast: One-minute protocol
●
Higher purity and recovery > 90% DNA
●
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and ethanol
●
High throughput: Compatible with many different automated liquid handling systems
A. Materials Required by the User
●
18.2 MΩ.cm, DNase/RNase-Free Ultrapure Water
●
Triton™ X-100, Sigma, Catalog No. T8787
●
Others
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Item
Adjustable Single and Multichannel Pipettes
Item
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
** IMPORTANT! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Adjustable Single and Multichannel Pipettes
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
! IMPORTANT ! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
B. Procedure
! Important !
1.
The following protocol is optimized for the efficient cleanup of 10μl DNA sample. The procedure may need to be optimized if an alternative reaction scale is used.
2.
Shake or vortex the bottle to completely resuspend the magnetic beads before using.
3.
Do not allow the magnetic beads to sit for more than two minutes before dispensing.
4.
Based on applications, the user should choose buffer conditions based on table 1. For example, if the sample does not contain detergent, add 1 μL of 1% Triton™ X-100 solution to a 10 μL sample (final concentration is 0.1%).
5.
Quantification of the nucleic acids: Use only fluorescence methods such as qPCR, Qubit, and Pico Green.
Table 1 – DNA Fragment Removal
Table 1 – DNA Fragment Removal
DNA
Buffer
+ 0.1%
Triton x-100
pH7.5
– 0.1%
Triton x-100
pH7.5
+ 0.1%
Triton x-100
pH 8.0
– 0.1%
Triton x-100
pH 8.0
+ 0.1%
Triton x-100
pH 8.8
– 0.1%
Triton x-100
pH 8.8
dsDNA
(100 bp)
No Removal
Removal
Removal
Removal
No Removal
Removal
dsDNA
(150 bp)
No Removal
Removal
No Removal
Removal
No Removal
Removal
dsDNA
(200 bp)
No Removal
Removal
No Removal
Removal
No Removal
Removal
dsDNA
(300 bp)
No Removal
No Removal
No Removal
No Removal
No Removal
No Removal
ssDNA
100 mer
Removal
Removal
Removal
Removal
Removal
Removal
Please Note:
dsDNA – Double-Stranded DNA; ssDNA – Single-stranded DNA
The assay was done by using the following conditions:
1. 10 mM Tris-HCl with or without 0.1% triton (final concentration) and three different: pH 7.5, pH 8.0 and pH 8.8
1.
Add 5 μL magnetic beads to the 10 μL DNA sample.
2.
If necessary, briefly centrifuge at 2500 rpm for 30 seconds to bring all contents to the bottom of the tube.
3.
Mix thoroughly for 1 minute by slowly pipetting up and down 25 times (one minute) or by vortex mixer for 5 minutes at 2500 rpm.
4.
If necessary, briefly centrifuge at 2500 rpm for 30 seconds to bring all contents to the bottom of the tube.
5.
Place the sample plate on the magnetic separation plate for 30 seconds or until the solution is clear to separate beads from the solution.
6.
Transfer the supernatant to a clean plate while the sample plate remains on the magnetic separation plate for downstream applications.
C. Troubleshooting
Problem
Low DNA Recovery
Probable Cause
Vertexing speed is too fast.
Vertexing time is too long.
Suggestion
Problem
Low DNA Recovery
Probable Cause
Using too many magnetic beads
Suggestion
Thoroughly resuspend the magnetic beads and use the correct amounts of the beads.
Problem
Failure to Remove Impurities.
Probable Cause
Used inappropriate PCR tubes or PCR plates
Suggestion
Make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates is ≥2.5mm.
Problem
Failure to Remove Impurities.
Probable Cause
Vortex speed is too slow, or vortex time is too short.
Suggestion
Problem
Failure to Remove Impurities.
Probable Cause
Using fewer magnetic beads
Suggestion
Thoroughly resuspend the magnetic beads and use the correct amounts of the beads.
Problem
Failure to Remove Impurities.
Probable Cause
Strong secondary structure of DNA fragments ( < 50bp dsDNA or < 100 mer ssDNA)
Suggestion
Denature the sample by heating it at 95°C for 2 min.
Problem
Failure to Remove Impurities.
Probable Cause
Too much primer, dimer, adaptor, free dye, and detergent
Suggestion
Problem
Probable Cause
Suggestion
Low DNA Recovery
Vertexing speed is too fast.
Vertexing time is too long.
Using too many magnetic beads
Thoroughly resuspend the magnetic beads and use the correct amounts of the beads.
Failure to Remove Impurities.
Used inappropriate PCR tubes or PCR plates
Make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates is ≥2.5mm.
Vortex speed is too slow, or vortex time is too short.
Using fewer magnetic beads
Thoroughly resuspend the magnetic beads and use the correct amounts of the beads.
Strong secondary structure of DNA fragments ( < 50bp dsDNA or < 100 mer ssDNA )
Denature the sample by heating it at 95°C for 2 min.
Too much primer, dimer, adaptor, free dye, and detergent
Magnetic Beads Make Things Simple