- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Components
Shipping conditions: At ambient temperature
Components
BcMag™ U-DNA Beads
10x Lysis Buffer (100mM Tris-HCl, PH 9.0)
Proteinase K
DTT
Proteinase K Suspension Buffer
Storage
4°C
4°C
-20°C
-20°C
4°C
Cat. No. AJ101 (50 Preps)
2.5 ml
0.6 ml
12.5 mg
15.4 mg
1.0 ml
Cat. No. AJ102 (100 Preps)
5.0 ml
1.2 ml
25 mg
30.8 mg
2.0 ml
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit components according to the table Above on arrival.
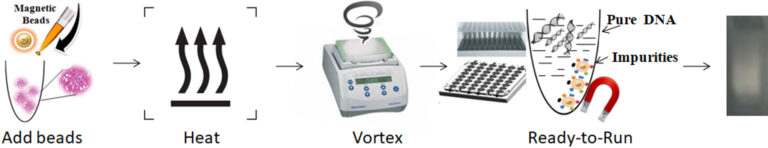
1.
To lyse the sample, add functional magnetic beads and proteinase K to the sample and incubate at 65°C.
2.
Vortex/pipette the beads with the sample to capture the PCR inhibitors.
3.
Separate the beads from the sample using a magnet.
4.
Aspirate the supernatant containing the pure, ready-to-use DNA/RNA.
●
Rapid and efficient purification protocol: without prior DNA isolation for subsequent use in direct workflows, No liquid transfer, and One-tube.
●
Ultrafast: Process 96 samples in less than an hour.
●
Highest nucleic acids recovery rates: Minimal loss of DNA during extraction
●
●
Highly improved active paraffin removal: No need for toxic organic solvents, such as xylene
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High throughput: Compatible with many different automated liquid handling systems.
The following protocol is an example. The protocol can be scaled up or down as needed.
It’s important to note that DNA isolated from FFPE samples has a lower molecular weight than DNA recovered from fresh or frozen samples. The degree of fragmentation is determined by the type and age of the sample, as well as the fixation conditions used. The kit is designed for the usage of FFPE mammalian tissue samples. It is not intended for use with non-FFPE tissue samples, such as fresh or frozen tissue samples or FFPE samples obtained from nonmammalian tissues.
Notes
●
DNA Yield: Varies (depends on sample size and type)
●
DNA Size: Varies (depends on the quality of starting material)
●
Since there is no concentration step in the protocol, the concentration of the nucleic acid depends on the quality and quantity of the sample used.
●
●
For long-term storage, store the extracted nucleic acids at -20°C.
Materials Required by the User
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-06)
Item
Adjustable Single and Multichannel pipettes
Item
Centrifuge with swinging bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-06)
Adjustable Single and Multichannel pipettes
Centrifuge with swinging bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
! IMPORTANT ! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
A. Premix Beads Solution Preparation
! IMPORTANT !
1.
Before pipetting, shake or Vortex the bottle to completely resuspend the Magnetic Beads.
2.
Do not allow the magnetic beads to sit for more than 2 minutes before dispensing.
3.
Proteinase K preparation: Provide protease K as lyophilized powder and dissolve at a 20 mg/ml concentration in Proteinase K Suspension Buffer. For example, 12.5 mg dissolved in 625 µl of Proteinase K Suspension Buffer. Divide the stock solution into small aliquots and store at -20°C. Each aliquot can be thawed and refrozen several times but should then be discarded.
4.
DTT solution preparation: Provide DTT as powder and dissolve at a concentration of 1M in ultrapure water. For example, 15.4 mg dissolved in 100µl ultrapure water. It is stable for years at -20°C. Prepare in small aliquots, thaw it on ice, and use and discard. Store them in the dark (wrapped in aluminum foil) at -20°C. Do not autoclave DTT or solutions containing it. Avoid multiple freeze-thaw cycles.
5.
Dilute DTT to a concentration of 10 mM from stock with ultrapure water and use it immediately. Discard unused DTT solution.
6.
Prepare a fresh Master Mix following Table 2 for the number of samples to be processed, plus 10% more (e.g., if you have 10 samples, prepare Master Mix for 11). Add the following components to the reservoir.
Table 2. Premix Beads solution
Components
BcMag™ U-DNA Beads
10x Lysis Buffer
Proteinase K (20mg/ml)
DTT (10 mM)
Sample
Ultrapure Water
Total
One Well ( 100 μL Reaction Volume)
50 μl
10 μl
12.5 μl
3 μl
x
x
100 μl
Components
B. Isolation Procedure
! IMPORTANT !
●
Pipet up and down premix beads solution in a reagent reservoir until the solution is homogeneous before dispensing.
●
Do not allow the magnetic beads to sit for more than 5 minutes before dispensing.
1.
Transfer the sample to a new well of a 96well PCR plate. The size of the FFPE slides was 2µm – 5µm. Excess wax was removed from the slide before collecting FFPE mammalian tissue sections, and the tissue was collected with a scalpel and transferred to a 0.2ml PCR tube). For Fine Aspiration Biopsy (FNA) sample, use 5 µL-10 µLsample.
Note: It is not designed for sample volumes larger than 2µm – 5µm. Only use sections that meet the size specification.
2.
Transfer 100µL premix beads solution to the sample. (Note. Use 50 µl premix beads solution for <2.5 µm FFPE tissue section)
3.
Mix the sample well by Vortex or pipetting.
4.
Place the PCR plate/tube into a thermocycler and incubate at:
a.
65°C for 60 minutes
b.
90°C for 90 minutes
5.
Remove the PCR plate/tube from the thermocycler and then mix the sample with beads by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 5 minutes (see picture).
6.
Centrifuge at 3500 rpm for 5 minutes.
7.
Place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear.
8.
Transfer the supernatant to a clean plate /tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications. Using 1-5 μl in a 25μl for qPCR.
C. Troubleshooting
Problem
Low DNA/RNA Recovery
Probable Cause
Suggestion
Problem
Ct Value Delays
Probable Cause
Suggestion
Problem
Probable Cause
Suggestion
Low DNA/RNA Recovery
Ct Value Delays
Magnetic Beads Make Things Simple