- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Components
Shipping conditions: At ambient temperature
Components
BcMag™ U-DNA Beads
10x Lysis Buffer (100mM Tris-HCl, PH 9.0)
Proteinase K
DTT
Proteinase K Suspension Buffer
Storage
4°C
4°C
-20°C
-20°C
4°C
Cat. No. AF101 (50 Preps)
2.5 ml
0.6 ml
12.5 mg
15.4 mg
1.0 ml
Cat. No. AF102 (100 Preps)
5.0 ml
1.2 ml
25 mg
30.8 mg
2.0 ml
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit components according to the table Above on arrival.
The BcMag™ One-Step Blood DNA Purification Kit is an innovative solution for rapid and efficient genomic DNA purification from various sources, such as whole blood, serum, plasma, and other bodily fluids. The kit uses a unique proprietary magnetic bead technology to lyse cells and remove impurities simultaneously in an aqueous buffer, preserving DNA integrity by avoiding harsh conditions like alkaline lysis and toxic chemicals.
Moreover, the magnetic beads eliminate PCR inhibitors from samples in a single step, which enhances DNA integrity, increases nucleic acid yields, and reduces DNA loss typically caused by the time-consuming “bind-wash-elute” procedure used in DNA purification methods. With the one-step purification technique, more than 96 samples can be processed simultaneously, producing pure DNA in under 30 minutes.
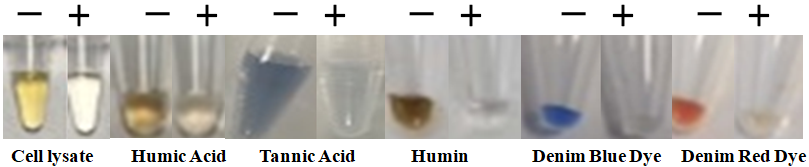
The resulting purified genomic DNA has the highest integrity and is suitable for a range of downstream applications, including qPCR, STR, and more. This cutting-edge technology provides a time-efficient, easy-to-use solution for researchers, enabling them to streamline their workflow and achieve reliable results without compromising DNA quality.
The specially designed magnetic beads can quickly capture the impurity once mixed with the cell lysate. Then, the magnetic beads-impurity complex is magnetically removed by a magnet while the pure DNA remains in the solution.
1.
Add magnetic beads to the sample.
2.
Mix the samples with the magnetic beads and proteinase K and heat to lyse the cells.
3.
Mix by vertexing/pipetting for the beads to capture the PCR inhibitors.
4.
Remove the beads with a magnet.
5.
Aspirate the supernatant containing the pure ready-to-use DNA.

The purified DNA is ready for downstream applications, such as PCR, qPCR, RT-PCR (reverse transcription-polymerase chain reaction), single-nucleotide polymorphism (SNP), short tandem repeat (STR) genotyping, genotyping, next-generation sequencing (NGS), veterinary genotyping, forensics, population studies, etc.
●
Rapid and efficient purification protocol: without prior DNA isolation for subsequent use in direct workflows, No liquid transfer, and One-tube.
●
Ultrafast: Process 96 samples in less than an hour.
●
Highest nucleic acids recovery rates: Minimal loss of DNA during extraction
●
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High throughput: Compatible with many different automated liquid handling systems.
The following protocol is an example. The protocol can be scaled up or down as needed.
Notes
●
DNA Yield: Varies (depends on sample size and type)
●
DNA Size: Varies (depends on the quality of starting material)
●
Since there is no concentration step in the protocol, the concentration of the nucleic acid depends on the quality and quantity of the sample used.
●
Quantification of the nucleic acids: Use only fluorescence methods such as qPCR, Qubit, and Pico Green.
●
●
For long-term storage, store the extracted nucleic acids at -20°C.
Materials Required by the User
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-06)
Item
Adjustable Single and Multichannel pipettes
Item
Centrifuge with swinging bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-06)
Adjustable Single and Multichannel pipettes
Centrifuge with swinging bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
! IMPORTANT ! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
A. Sample Collection
Handling Samples
Follow these general guidelines when handling forensic samples:
●
When possible and appropriate, cut the sample into small pieces to facilitate processing.
●
Avoid overloading the sample tube to allow efficient mixing of Lysis Mix with the sample.
●
When dealing with blood-stained items, ensure to use a minimum sample (≤ 4 μl blood spot). Processing large, heavily blood-stained items may contaminate the purified DNA with heme.
Sample
whole blood
Example sample input
serum, plasma
other body fluids
20µl
Blood swabs
Up to 3 mg (equal to ≤ 4 μl blood spot) from the dried blood spot using scissors and forcipes.
Body fluids (saliva, semen, blood) on dye denim and other fabrics
IMPORTANT: For the best results, punch in the center of the area where the sample was applied
storage cards
B. Premix Beads Solution Preparation
! IMPORTANT !
1.
Before pipetting, shake or Vortex the bottle to completely resuspend the Magnetic Beads.
2.
Do not allow the magnetic beads to sit for more than 2 minutes before dispensing.
3.
Proteinase K preparation: Provide protease K as lyophilized powder and dissolve at a 20 mg/ml concentration in Proteinase K Suspension Buffer. For example, 12.5 mg dissolved in 625 μl of Proteinase K Suspension Buffer. Divide the stock solution into small aliquots and store at -20°C. Each aliquot can be thawed and refrozen several times but should then be discarded.
4.
DTT solution preparation: Provide DTT as powder and dissolve at a concentration of 1M in Ultrapure water. For example, 15.4 mg dissolved in 100μl Ultrapure water. It is stable for years at -20°C. Prepare in small aliquots, thaw it on ice, and use and discard. Store them in the dark (wrapped in aluminum foil) at -20°C. Do not autoclave DTT or solutions containing it. Avoid multiple freeze-thaw cycles.
5.
Dilute DTT to a concentration of 10 mM from stock with Ultrapure water and use it immediately. Discard unused DTT solution.
6.
Prepare a fresh Master Mix following Table 2 for the number of samples to be processed, plus 10% more (e.g., if you have 10 samples, prepare Master Mix for 11). Add the following components to the reservoir.
Table 2. Premix Beads solution
Components
BcMag™ U-DNA Beads
10x Lysis Buffer
Proteinase K (20mg/ml)
DTT (10 mM)
Sample
Ultrapure Water
Total
One Well ( 100 μL Reaction Volume)
50 μl
10 μl
12.5 μl
3 μl
x
x
100 μl
Components
C. Isolation Procedure
! IMPORTANT !
●
Pipet up and down premix beads solution in a reagent reservoir until the solution is homogeneous before dispensing.
●
Do not allow the magnetic beads to sit for more than 5 minutes before dispensing.
1.
Transfer 100μl premix beads solution to the sample (except dye denim and other fabrics, whose purification goes to section D) to a new well of 96well PCR plate or 0.2ml PCR tube and add the sample.
2.
Mix the sample well by Vortex or pipetting.
3.
Place the PCR plate/tube into a thermocycler and incubate at:
a.
65°C for 15 minutes
b.
80°C for 10 minutes
4.
Remove the PCR plate/tube from the thermocycler and then mix the sample with beads by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 5 minutes (see picture).
5.
Centrifuge at 3500 rpm for 5 minutes.
6.
Place the sample plate / tube on the magnetic separation plate for 30 seconds or until the solution is clear.
7.
Transfer the supernatant to a clean plate / tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications. Using 1-5 μl in a 25μl RT-PCR or qPCR.
D. Isolation procedure for samples of dye denim and other fabrics
1.
Transfer 100μl premix beads solution to the dye denim and other fabrics to a new well of 96well PCR plate or 0.2ml PCR tube and add the sample.
2.
Mix the sample well by Vortex or pipetting.
3.
Place the PCR plate/tube into a thermocycler and incubate at 60°C for 30 minutes.
4.
Remove the PCR plate/tube from the thermocycler and place the sample plate/ tube on the magnetic separation plate for 30 seconds or until the solution is clear.
5.
Rolling against the tube sides, press the sample against the side to squeeze as much of the liquid as possible and, simultaneously, leave beads as much as possible by forceps.
6.
Remove the dye denim or fabrics and transfer supernatant with beads to a new PCR tube.
7.
Place the PCR plate/tube into a thermocycler and incubate at 80°C for 10 minutes.
8.
Remove the PCR plate/tube from the thermocycler, mix the sample with beads by slowly pipetting up and down 20-25 times, or Vortex the sample at 2000 rpm for 5 minutes (see picture).

9.
Centrifuge at 3500 rpm for 5 minutes.
10.
Place the sample plate / tube on the magnetic separation plate for 30 seconds or until the solution is clear.
11.
Transfer the supernatant to a clean plate / tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications. Using 1-5 μl in a 25μl RT-PCR or qPCR.
E. Troubleshooting
Problem
Low DNA/RNA Recovery
Probable Cause
Poor starting sample material
Suggestion
Problem
Ct Value Delays
Probable Cause
Too many PCR inhibitors in the sample.
Suggestion
Problem
Ct Value Delays
Probable Cause
Recovery DNA is so low.
Suggestion
Problem
Probable Cause
Suggestion
Low DNA/RNA Recovery
Poor starting sample material
Ct Value Delays
Too many PCR inhibitors in the sample.
Recovery DNA is so low.
Magnetic Beads Make Things Simple