- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
Specification
Composition
Magnetic grafted with a high density of iodoacetyl group
Number of Beads
~ 1.68 x 109 beads/mg (1μm beads)
~ 5 x 107 beads /mg (5μm beads)
Stability
Short Term (<1 hour): pH 3-11; Long-Term: pH 4-10
Temperature: 4°C -140°C; Most organic solvents
Magnetization
~40-45 EMU/g
Type of Magnetization
Superparamagnetic
Formulation
Lyophilized Powder
Functional Group Density
1μm Magnetic Beads
~200 μmole / g of Beads
5μm Magnetic Beads
~180 μmole / g of Beads
1μm Long-Arm Magnetic Beads
~160 μmole / g of Beads
5μm Long-Arm Magnetic Beads
~130 μmole / g of Beads
Storage
Store beads at -20°C protected from light and free of moisture upon receipt
It is frequently helpful to immobilize affinity ligands via functional groups other than amines. The thiol group, in particular, can be employed to guide coupling processes away from active centers or binding sites on specific protein molecules.
Cysteines are frequently bonded between their side chains via disulfide bonds (-S-S-) as part of a protein’s secondary or tertiary structure. The side chain of cysteine contains sulfhydryls (-SH) (Cys, C). These must be converted to sulfhydryls before being immobilized. Thiol groups (sulfhydryls) can be found naturally in proteins or introduced through the reduction of disulfides or using various thiolation reagents.
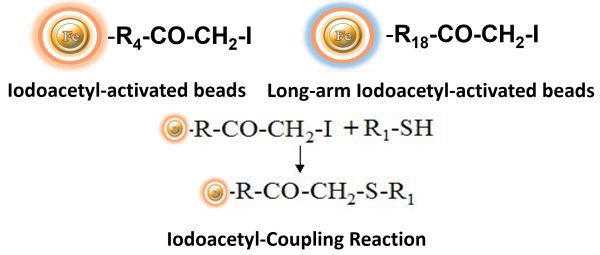
BcMag™ Iodoacetyl-activated Magnetic Beads are uniform, silica-based superparamagnetic beads coated with a high density of Iodoacetyl functional groups on the surface. It is designed to enable fast, efficient, and covalent immobilization of protein, peptides, and other ligands through their sulfhydryl groups (-SH) for affinity purification procedures. At physiological to alkaline circumstances (pH 7.2 to 9) ) in either aqueous or organic solvents with 20- 30% DMSO or DMF, iodoacetyl-activated supports react with sulfhydryl groups, resulting in stable thioether bonds. These reactions are often carried out in the dark to prevent the formation of free iodine, which can react with tyrosine, histidine, and tryptophan residues. The hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. BcMag™ Iodoacetyl-activated Magnetic Beads are most suitable for conjugation of a larger protein. BcMagTM Long-arm Iodoacetyl-activated Magnetic Beads are recommended to conjugate small peptides because the long-arm (20-atom) hydrophilic linker may reduce steric hindrance.
Conjugation Protocol
Note:
●
This protocol can be scaled up as needed. We strongly recommended titration to optimize the number of beads used for each application.
Materials Required
●
Coupling Buffer
1.
Soluble coupling buffer : 50 mM Tris, 5 mM EDTA-Na, pH 8.5
b.
Insoluble coupling buffer: 50 mM Tris, 5 mM EDTA-Na, pH 8.5, 20- 30% DMSO or DMF or 6 M guanidine•HCl
●
●
L-Cysteine•HCl
●
TCEP (tris(2-carboxyethyl)phosphine)
●
Phosphate buffered saline (PBS)
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
1. BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
2. BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
3. BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
4. BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
5. BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
A.
Ligand preparation
Note:
●
Ensure that ligands have free (reduced) sulfhydryls. If free sulfhydryl groups are not available, use a reducing agent such as DTT (dithiothreitol), TCEP (tris(2-carboxyethyl)phosphine), or 2-MEA (2-Mercaptoethylamine•HCl) to treat ligands followed by desalting or dialysis to remove the reducing agent.
●
Newly Synthesized peptides may be directly used for coupling if used immediately after reconstitution.
●
For protein, treat protein with 5-10 mM TCEP solution for 30 minutes at room temperature, followed by dialysis or a desalting column. For IgG antibodies, 2-MEA is recommended due to its Selective reduction of hinge-region disulfide bonds.
●
If the sample contains reducing agents with free sulfhydryls (e.g., 2-mercaptoethanol or DTT), these agents must be completely removed by dialysis or desalting.
1.
Dissolve 1-10mg protein/peptide in 1ml soluble coupling buffer if soluble. If insoluble, dissolve in 1ml insoluble coupling buffer.
2.
If samples have already been suspended in other buffers, dilute samples with an equal volume of coupling buffer.
B.
Magnetic beads preparation
1.
Prepare 3% magnetic beads with 100% Acetone (30 mg/ml).
Note: Store the unused beads in acetone solution at 4°C. It has been stable for over a year.
2.
Transfer 100 μl (3mg) magnetic beads to a centrifuge tube.
3.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Remove the tube from the rack and resuspend the beads with 1 ml coupling buffer by vortex for 30 seconds.
4.
Repeat step 3 two times.
5.
Remove the supernatant, and the washed beads are ready for coupling.
Note: Once rehydrated using the coupling buffer, use the bead as soon as possible due to the stability of the functional group.
C.
Coupling
1.
Add 100 μl of ligand to the washed beads, mix well and incubate the sample in the dark at room temperature overnight with good mixing (end-over-end).
2.
Wash the magnetic beads with 1ml coupling buffer four times.
3.
Block the excess active groups on the beads by suspending the beads in 1ml Coupling buffer containing 8mg L-Cysteine•HCl and incubate 30-60 minutes at room temperature with gentle rotation.
4.
Wash the beads with 1ml washing buffer four times.
5.
Resuspend the beads in PBS buffer containing 0.05% sodium azide and store them at 4°C.
D.
General Affinity Purification Protocol
Note:
●
This protocol is a general affinity purification procedure. Designing a universal protocol for all protein purification is impossible because no two proteins are precisely alike. To obtain the best results, each user must determine the optimal working conditions for the purification of the individual target protein.
●
We strongly recommended titration to optimize the number of beads used for each application based on the amount of the target protein in the crude sample. Too many magnetic beads used will cause higher backgrounds, while too few beads used will cause lower yields. Each mg of magnetic beads typically binds to 10-20 μg of the target protein.
1.
Transfer the optimal amount of the beads to a centrifuge tube. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
2.
Remove the tube and wash the beads with 5-bed volumes of PBS buffer by vortex for 30 seconds. Leave the tube at room temperature for 1-3 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Repeat step 2 two times.
4.
Add washed beads to the crude sample containing the target protein and incubate at room or desired temperature for 1-2 hours (Lower temperatures require longer incubation time).
Note: Strongly recommended to perform a titration to optimize incubation time. More prolonged incubation may cause higher background.
5.
Note: Adding a higher concentration of salts, nonionic detergent, and reducing reagent may reduce the nonspecific background. For example, adding NaCl (up to 1-1.5 M), 0.1-0.5% nonionic detergents such as Triton X 100 or Tween 20, and a reducing reagent such as DTT or TCEP (we usually use 3mM) to the washing buffer.
6.
Elute the target protein by appropriate methods such as low pH (2-4), high pH (10-12), high salt, high temperature, affinity elution, or boiling in an SDS-PAGE sample buffer.
Magnetic Beads Make Things Simple