- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Components
Shipping conditions: At ambient temperature
Components
BcMag™ HO-DNA Beads
10x Lysis Buffer
1x Elution Buffer
Proteinase K
Proteinase K Suspension Buffer
SDS
DTT
Storage
4°C
4°C
4°C
-20°C
4°C
Room Temperature
-20°C
Cat. No. AC101 (50 Preps)
1.0 ml
5.0 ml
0.5 ml
15 mg
1.0 ml
2.5 ml
2.5 ml
Cat. No. AC102 (100 Preps)
2.0 ml
10 ml
1.0 ml
30 mg
2.0 ml
5.0 ml
5.0 ml
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit components according to the table Above on arrival.
The BcMag™ Cell-Free DNA Purification Kit is a highly efficient and precise system for extracting cell-free DNA (cfDNA) from human plasma. With its unique proprietary magnetic beads and optimized buffer system, the kit offers gentle lysis conditions that protect the integrity of the DNA and avoid harsh treatments.
Designed for both manual and automatic processing, the kit provides flexibility for researchers, allowing for the processing of multiple samples simultaneously with an automation system or low-volume samples manually using a magnetic stand. This makes it ideal for large-scale studies, where time and effort are crucial.
The use of magnetic beads in the extraction process eliminates the risk of clogging, a common issue in protein-rich plasma samples. The beads are also more efficient in binding DNA than glass fiber filters, leading to higher and more consistent yields. The kit can handle input volumes ranging from 100 µL to 10 mL of plasma and elution quantities ranging from 15 µL to 50 µL, making it an excellent solution for research projects of any size.
The BcMag™ Cell-Free DNA Purification Kit is also phenol-free, ensuring the purified cfDNA is of the highest quality and integrity. The kit is automation-ready, allowing for easy and speedy processing of multiple samples with minimal effort. With its unparalleled efficiency and precision, the BcMag™ Cell-Free DNA Purification Kit is an excellent choice for any researcher looking for high-quality yields and reliable results.
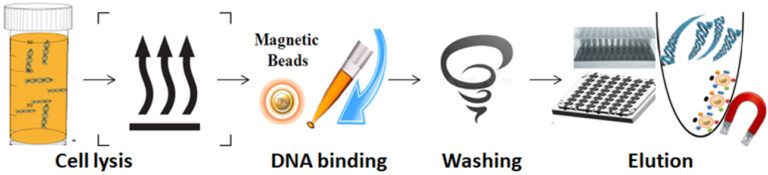
1.
Add lysis buffer and proteinase K to the sample to lyse the bone and incubate at 65°C.
2.
Add functional magnetic beads and vortex/pipette the beads with the sample to the DNA.
3.
Wash the beads.
4.
Separate the beads from the sample using a magnet.
5.
Elute DNA from the beads.
●
Scalable format for processing both high and low-volume samples
●
Flexible design for manual and automatic cfDNA isolation
●
Automation-ready and phenol-free extraction process
●
Input volumes from 100 µL to 10 mL of plasma
●
Elution quantities ranging from 15 µL to 50 µL
The following protocol is an example. The protocol can be scaled up or down as needed.
Notes
●
DNA yield: The yield and quality of circulating DNA are highly influenced by a blood sample, processing, storage, and plasma preparation. It is strongly advised to carry out these stages as uniformly as possible to obtain maximum reproducibility—cell-free DNA yields between 1 and 100 ng per ml of plasma. To quantify purified cfDNA, we recommend utilizing the QubitTM dsDNA High Sensitivity Assay.
●
DNA Size: Varies (depends on the quality of starting material)
●
For long-term storage, store the extracted nucleic acids at -20°C.
●
Proteinase K preparation: Provide protease K as lyophilized powder and dissolve at a 20 mg/ml concentration in Proteinase K Suspension Buffer. Divide the stock solution into small aliquots and store at -20°C. Each aliquot can be thawed and refrozen several times but should then be discarded.
●
DTT solution preparation: Provide DTT as powder and dissolve at a concentration of 20% in dH2O. It is stable for years at -20°C. Prepare in small aliquots, thaw it on ice, and use and discard. Store them in the dark (wrapped in aluminum foil) at -20°C. Do not autoclave DTT or solutions containing it. Avoid multiple freeze-thaw cycles.
A. Materials Required by the User
●
95–100% ethanol
●
Isopropanol
●
65°C Incubator chamber
●
Microcentrifuge tubes
●
Aerosol-resistant micropipette tip
●
Magnetic rack: Based on sample volume, the user can choose one of the following magnetic Racks:
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
BcMag™Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
B. Sample Preparation
The yield and quality of circulating DNA are highly influenced by a blood sample, processing, storage, and plasma preparation. It is strongly advised to carry out these stages as uniformly as possible in order to obtain maximum reproducibility cell-free DNA yields between 1 and 100 ng per ml of plasma. To quantify purified cfDNA, we recommend utilizing the QubitTM dsDNA High Sensitivity Assay.
Preparation of plasma
1.
Centrifuge fresh blood sample for 10 minutes at 2,000 x g.
2.
Transfer the plasma into a new tube without disturbing the sedimented cells.
3.
Centrifuge the plasma samples at 16,000 × g for 10 minutes at 4°C.
4.
Freeze plasma at -20 °C until DNA isolation.
5.
Prior to DNA isolation, thaw frozen plasma samples and centrifuge for 10 minutes at 16,000 x g at 4°C in a microcentrifuge for small plasma volumes or 30 minutes at 4,500 x g at 4°C in a tabletop centrifuge for larger plasma volumes to remove remaining cells, cell debris, and particulate matter. Make use of the supernatant to isolate DNA.
C. Purification
1.
Prepare the plasma samples by thawing frozen plasma at room temperature. Centrifuge plasma for 5 minutes at 12000x g to remove any blood and cell debris.
2.
Add the following components to a tube in the order indicated and vortexing for 1 min to mix well. Briefly spin down.
Components
Plasma
10x Lysis buffer
Proteinase K (20mg/ml)
DTT (20%)
0.1 ml
10 μL
15 μL
5 μL
5 μL
0.5 ml
50 μL
75 μL
25μL
25 μL
1ml
100 μL
150 μL
50μL
50 μL
2ml
200 μL
300 μL
100μL
100 μL
5ml
500μL
750μL
250 μL
250 μL
10ml
1000μL
1.5 mL
500 μL
500 μL
[1] Do not add SDS directly to the Proteinase K solution to avoid inactivation of the Proteinase K.
3.
Vertexing for 1 min to mix well. Briefly spin down.
4.
Incubate at 65°C for 30 min.
5.
After the 30-minute incubation period, place the tubes containing the plasma sample on ice for 5 minutes to cool them to room temperature.
6.
Centrifuge for 10 minutes at 16,000 x g and transfer the supernatant to a new appropriate centrifuge tube
7.
Prepare the Binding Solution/Beads/Isopropanol Mix according to the following table and mix thoroughly.
8.
Add the appropriate amount of beads and Isopropyl based on the following table.
Plasma
BcMag™ HO-DNA Beads**
Isopropyl alcohol
0.1 ml
5 μL
82 μL
0.5 ml
15 μL
410 μL
1 ml
30 μL
820 μL
2 ml
60 μL
1.64 mL
5 ml
100 μL
4.1 mL
10 ml
200 μL
8.2 mL
9.
Vigorously shake the bottle until the magnetic beads become homogeneous before dispensing. Do not allow the beads to sit for more than 2 minutes before dispensing. Resuspend the magnetic beads every 2 minutes.
10.
Place the tube on the magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack. Add 500μL-1000 μL of 85% Ethanol and mix by vortex for 5 minutes or pipetting 25-35 times to wash the beads. Place the tube on the magnetic Rack for 1-3 minutes and remove the supernatant completely while the tube remains on the Rack.
11.
Repeat step (10) two times.
12.
Remove the tube from the magnetic Rack and let the beads air dry for 10-30 minutes to evaporate the ethanol completely.
13.
Add an appropriate amount of 1x Elution buffer based on the following table and mix by vortex for 5 minutes or pipetting 25-35 times to elute the DNA from the beads.
BcMag™ HO-DNA Beads**
1x Elution Buffer
5 μL
10 μL
15 μL
10 μL
30 μL
15 μL
60 μL
25 μL
100 μL
50 μL
200 μL
75 μL
14.
Place the tube on the magnetic Rack for 1-3 minutes and transfer the supernatant to a new centrifuge tube. The eluted DNA should be stored at -20°C.
Magnetic Beads Make Things Simple