- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Specification
Composition
Magnetic beads grafted with C8 alkyl groups
Number of Beads
~ 1.68 x 109 beads/mg (1μm beads)
~ 5 x 107 beads /mg (5μm beads)
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.0 g/ml
Stability
Most organic solvents
Formulation
Lyophilized Powder
Binding Capacity
1 μm beads >20 μg protein/mg of Beads
5 μm beads >18 μg protein/mg of Beads
Storage
Store at 4°C upon receipt.
The hydrophobic interaction chromatography (HIC) technique is a perplexing yet intriguing method for separating macromolecules. It involves a reversible interaction between the external hydrophobic region of a biological macromolecule and the hydrophobic ligand of a HIC medium, which could be phenyl, octyl, or butyl. This interaction is heavily influenced by the salt concentration in a buffer. A high salt concentration enhances the interaction, while a low salt concentration reduces it. Due to this salt concentration dependence, proteins with lower hydrophobicity levels elute first, while those with more hydrophobicity levels elute last.
HIC is a chromatography method that is quite enigmatic compared to other techniques. It is, however, a popular method used to separate and purify proteins and peptides at analytical and preparatory scales. The reason for its popularity is that it employs a less denaturing environment.
BcMag™ C-8 Magnetic Beads are renowned for their remarkable uniformity and superparamagnetic properties. These beads possess hydrophobic groups on their surface that enable them to function effectively as a chromatographic matrix. They are specifically designed to facilitate the manual or automatic purification, desalination, and concentration of peptides or proteins in the femtomolar to picomolar range. This eliminates the need for repetitive and time-consuming procedures such as pipetting and centrifugation.
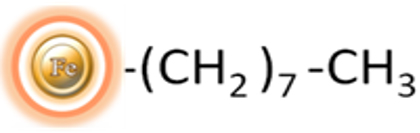
Note:
●
To achieve maximum binding to the hydrophobic magnetic beads, TFA (trifluoroacetic acid) or other ion-pairing agents should be between 0.1%–1.0% at a pH of <4. The solvents should be completely removed if samples contain excess organic solvents such as methanol or acetonitrile (ACN). Samples can be dried in a vacuum evaporator and resuspended in sample buffer (below). To optimize binding, detergents in samples should be diluted with 0.1% TFA till SDS <0.1%, or Triton ® <1%, or Tween® <0.5%.
●
To avoid excessive beads drying between steps, the entire procedure should be carried out in a timely manner.
●
The amount of beads used in each application should be empirically titrated. The volumes can be scaled up or down accordingly. We recommend using 10 μl (0.5 mg) hydrophobic magnetic beads to bind ~ 10 μg protein and 5μl elution buffer for 0.5 mg beads.
●
Users are encouraged to determine the optimal working conditions based on the protocol and suggestions described in the Troubleshooting section to get the best results.
Materials Required
●
Buffers
Equilibration buffer: 0.5% TFA (trifluoroacetic acid) in 5% ACN (acetonitrile)
Sample Binding Buffer: 2% TFA in 5% ACN
Washing buffer: 0.5 % TFA in 5% ACN
Elution Buffer: 70% ACN
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
Procedure
A.
Magnetic Beads Preparation
1.
Weight and suspend 50 mg beads with 1ml of 50% methanol.
2.
Transfer 10μl (50 mg/ml) of completely suspended magnetic beads to a microcentrifuge tube.
3.
Place the tube onto a magnetic rack for 1-3 minutes until the supernatant is clear.
4.
Wash the beads with 1 ml of Equilibration buffer by a magnetic rack.
5.
Aspirate and discard the supernatant with a pipette while the tube remains in the rack.
B.
Sample Binding
1.
Mix sample (~10μg protein/ peptide) with 1/3 volume of Sample Binding Buffer and add to the tube containing the beads.
2.
Thoroughly mix beads and sample using a pipette and leave at room temperature for 2 minutes to allow proteins to bind to the beads.
3.
Place the tube onto the magnetic rack for 1-3 minutes (no longer than 3 minutes) until the supernatant is clear. Aspirate and discard the supernatant with a pipette while the tube remains in the rack.
4.
Remove the tube from the rack and resuspend the beads with 100μl washing buffer.
5.
Place the tube onto the magnetic rack for 1-3 minutes until the supernatant is clear. Aspirate and discard the supernatant with a pipette while the tube remains in the rack.
6.
Repeat steps 2 to 4 for four times.
C.
Elution
1.
Remove the tube from the rack, add 5μl elution buffer, resuspend the beads and incubate for 2 minutes at room temperature.
2.
Place the tube on the magnetic rack for 1-3 minutes and transfer the supernatant containing the eluted protein to a new tube. (User should optimize elution conditions for individual proteins by adjusting acetonitrile concentrations, such as 20%, 50%, 80%).
3.
For MALDI-MS analysis, mix 1μl of the eluate with 1μl of matrix solution and spot 0.5μl onto a MALADI-MS target plate.
Troubleshooting
Problem
Poor absorption of proteins/Peptides to beads.
Probable Cause
Hydrophobic interaction is not strong enough.
Suggestion
Increase the NaCl concentration (up to 0.2 M) used during adsorption.
Problem
Poor absorption of proteins/Peptides to beads.
Probable Cause
Biomolecules are not completely solubilized in the sample buffer.
Suggestion
Use denaturing conditions during adsorption. Add Guanidine HCl to the sample to achieve a final concentration between 1– 6 M.
Problem
Poor absorption of proteins/Peptides to beads.
Probable Cause
The sample’s chemical properties do not support hydrophobic interaction with reverse-phase beads.
Suggestion
Choose suitable reverse phase beads for your sample.
Problem
Poor elution
Probable Cause
Hydrophobic interaction is too strong.
Suggestion
Increase the acetonitrile concentration used during elution. Decrease the NaCl concentration used during adsorption.
Problem
Poor elution
Probable Cause
Proteins/peptides are not readily soluble in organic solutions.
Suggestion
Decrease the organic solvent concentration used during elution.
Problem
Poor elution
Probable Cause
Protein-bound too tightly to beads
Suggestion
Choose more suitable reverse phase beads for your sample.
Problem
Poor yield
Probable Cause
The quantity of protein or peptides of interest in the sample is too low.
Suggestion
Problem
Probable Cause
Suggestions
Poor absorption of proteins/Peptides to beads.
Hydrophobic interaction is not strong enough.
Increase the NaCl concentration (up to 0.2 M) used during adsorption.
Biomolecules are not completely solubilized in the sample buffer.
Use denaturing conditions during adsorption. Add Guanidine HCl to the sample to achieve a final concentration between 1– 6 M.
The sample’s chemical properties do not support hydrophobic interaction with reverse-phase beads.
Choose suitable reverse phase beads for your sample.
Poor elution
Hydrophobic interaction is too strong.
Increase the acetonitrile concentration used during elution. Decrease the NaCl concentration used during adsorption.
Proteins/peptides are not readily soluble in organic solutions.
Decrease the organic solvent concentration used during elution.
Protein-bound too tightly to beads
Choose more suitable reverse phase beads for your sample.
Poor yield
The quantity of protein or peptides of interest in the sample is too low.
1.
Edelmann MJ. Strong cation exchange chromatography in analysis of posttranslational modifications: innovations and perspectives. J Biomed Biotechnol. 2011;2011:936508.
2.
Stone MT, Cotoni KA, Stoner JL. Cation exchange frontal chromatography for the removal of monoclonal antibody aggregates. J Chromatogr A. 2019 Aug 16;1599:152-160.
3.
Herciková J, Spálovská D, Frühauf P, Izák P, Lindner W, Kohout M. Design and synthesis of naphthalene-based chiral strong cation exchangers and their application for chiral separation of basic drugs. J Sep Sci. 2021 Sep;44(18):3348-3356.
4.
Janakiraman VN, Solé M, Maria S, Pezzini J, Cabanne C, Santarelli X. Comparative study of strong cation exchangers: Structure-related chromatographic performances. J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Mar 30;1080:1-10.
5.
Wang F, Dong J, Jiang X, Ye M, Zou H. Capillary trap column with strong cation-exchange monolith for automated shotgun proteome analysis. Anal Chem. 2007 Sep 1;79(17):6599-606.
6.
Das S, Bosley AD, Ye X, Chan KC, Chu I, Green JE, Issaq HJ, Veenstra TD, Andresson T. Comparison of strong cation exchange and SDS-PAGE fractionation for analysis of multiprotein complexes. J Proteome Res. 2010 Dec 3;9(12):6696-704.
7.
Steinebach F, Wälchli R, Pfister D, Morbidelli M. Adsorption Behavior of Charge Isoforms of Monoclonal Antibodies on Strong Cation Exchangers. Biotechnol J. 2017 Dec;12(12).
Magnetic Beads Make Things Simple