- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Kit Components
CA103
100 mg
2.5µm Amine-terminated magnetic beads
5 ml
2x Suspension Buffer
10 ml
10x Washing Buffer
0.15 g
CA104
200 mg
2.5µm Amine-terminated magnetic beads
10 ml
2x Suspension Buffer
20 ml
10x Washing Buffer
0.3 g
Specificities
Composition
Amine-terminated magnetic beads
Bead Size
2.5µm diameter
Number of Beads
~ 1.68 x 109 beads/mg
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.5 g/ml
Stability
pH 4-10
Concentration
Binding Capacity
>10 µg Oligo-DNA (25 nucleotides)/mg
Storage
Store at 4°C upon receipt
Many DNA microarray devices and chips have been created to detect DNA or RNA via solid phase hybridization. These biochips are generally based on the immobilization of DNA on a solid surface. They can collect and bind complementary DNA/RNA of interest from a tiny amount of samples. Immobilization of DNA/RNA on solid surfaces has grown in importance due to its applications in forensic science, environmental investigations, diagnosis and gene expression analysis, detection of single nucleotide polymorphisms and mutations, DNA sequencing or genetic disease diagnosis, gene expression analysis, detection of single nucleotide polymorphisms and mutations.
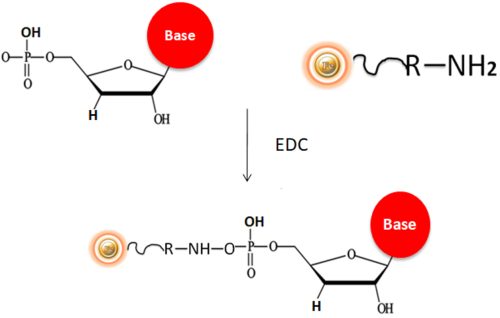
BcMag™ Quick DNA-RNA Conjugation Kit is intended to quickly and efficiently immobilize DNA, RNA, or phosphate-modified RNA/DNA oligos to our proprietary magnetic beads. For a more secure attachment, the kit is designed to use cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) to covalently immobilize 5′ phosphate group of DNA/RNA to amine-terminated magnetic beads (Fig.1). Our proprietary neutral coupling buffer makes the conjugation very efficient (>85%). The conjugated DNA is very stable and can be used for many downstream applications.
●
Quick, Easy, and one-step protocol
●
Neutral linkage—forms neutral amide bonds between phosphate and amine
●
Stable covalent bond with minimal ligand leakage
●
High immobilization efficiency
●
Scalable – Easily adjusts for sample size and automation
●
Reproducible results
The protocol can be scaled appropriately up or down.
Materials Required
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following magnetic Racks:
For larger scale purification, Ceramic magnets Block for large scale purification (6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8)
●
Corning 430825 cell culture flask for large-scale purification (Cole-Parmer, Cat. No. EW-01936-22)
●
Mini BlotBoy 3D Rocker, fixed speed, small 10″ x 7.5″ platform w/ flat mat (Benchmark Scientific, Inc. Cat. No. B3D1008) or compatible
A. DNA/RNA Sample Preparation
Note:
a.
All the samples (DNA/RNA) can not be suspended in TE or amine-containing buffer because it will reduce coupling efficiency.
b.
All the samples (DNA/RNA) must have a phosphate group at the 5′ end. A commercial oligo synthesis company can provide such service or treat oligo-DNA with T4 DNA Kinase for oligo-DNA. The oligo-DNA should be purified by standard desalting or other methods.
c.
Amplified PCR products must be purified by stander procedure before coupling.
d.
< 1kb for all DNA/RNA samples is preferred for coupling.
1.
All the samples (DNA/RNA) should be suspended in ultrapure water or 1x Suspension Buffer at a concentration of 5-10 µg/ul.
(Optional: Aspirate 5-10 µl sample, transfer to a new centrifuge tube, and label the tube as C1)
B. Coupling Buffer Preparation
1.
Prepare coupling buffer by adding 19 mg EDC to 1ml of 1x suspension buffer (Coupling buffer must be prepared fresh immediately before use)
C. Coupling
1.
Shake the bottle to resuspend the Magnetic Beads completely.
2.
Transfer 1ml magnetic beads (20 mg/ml) to a tube. Place the tube on the magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack.
3.
Remove the tube from the Rack and resuspend the beads thoroughly with a 1ml Suspension Buffer. Place the tube on the magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack.
4.
Repeat step 3 once.
5.
Remove the tube from the Rack and resuspend the beads thoroughly with a 200µl Coupling Buffer. Mix the magnetic beads with 100-200 µg DNA/RNA prepared from A1.
6.
Incubate the beads overnight at 50° C with continuous rotation.
7.
Place the tube on a magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack (Optional: Aspirate 5-10 µl supernatant, transfer to a new centrifuge tube and label the tube as C2).
8.
Wash the beads three times with 1 ml of Washing Buffer at room temperature and twice with ultrapure water at 65° C.
9.
Resuspend the beads at 5 mg/ml in PBS buffer containing 0.2% NaN3 and store at 4° C.
D. Coupling Efficiency Calculation
1.
Measure OD at A260
Coupling Efficiency (%) = [(C1-C2)/C1] x 100%
C1: A260 Pre-Coupling DNA/RNA Solution x dilution factor; C2: A260 post-Coupling DNA/RNA Solution.
2.
Using fluorescent dye to quantify C1 and C2.
1.
Ferrier DC, Shaver MP, Hands PJW. Micro- and nano-structure-based oligonucleotide sensors. Biosens Bioelectron. 2015 Jun 15;68:798-810.
2.
Sethi D, Gandhi RP, Kuma P, Gupta KC. Chemical strategies for immobilization of oligonucleotides. Biotechnol J. 2009 Nov;4(11):1513-29.
3.
Zuo P, Ye BC. A novel immobilization strategy using oligonucleotide as linker for small molecule microarrays construction. Biosens Bioelectron. 2008 Jun 15;23(11):1694-700.
Magnetic Beads Make Things Simple