- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Specification
Composition
Magnetic Bead grafted with TCEP group on the surface
Magnetization
~40 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.0 g/ml
Formulation
Lyophilized Powder
Loading
TCEP concentration > 12 mM /ml
Storage
Store at 4°C from light upon receipt
BcMag™ Immobilized TCEP Disulfide Reducing Kit (TCEP reducing magnetic resins) uses specifically designed magnetic beads to efficiently reduce disulfide bonds in proteins, peptides, and other disulfide bond-containing molecules (Fig.1). TCEP ([tris(2-carboxyethyl) phosphine] reducing magnetic resins avoid the need for time-consuming and resin,spin column and gravity-flow column procedures to separate the reduced sample from the reducing agent.
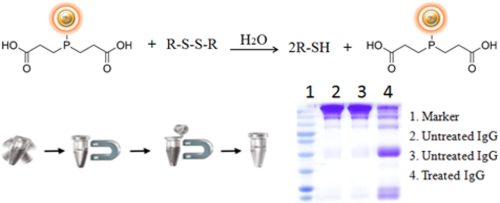
Immobilized TCEP Disulfide Reducing Beads allow for sample reduction and quick recovery of reducing agent-free samples. TCEP is an effective disulfide bond reducer in proteins, peptides, and other disulfide bond-containing molecules and is relatively unreactive to other functional groups. The trialkylphosphine TCEP is stable in aqueous solutions and does not oxidize as quickly as other reducing agents such as dithiotreitol (DTT) and -mercaptoethanol (BME). TCEP has little effect on common sulfhydryl-reactive chemicals (e.g., maleimide crosslinkers). Nonetheless, many protocols demand that the reduced sample be recovered separately from the reducing agent.
Magnetic resins have significant advantages over traditional chromatography, such as column, agarose, or non-magnetic resin. The magnetic bead-based format enables rapid high-yield processing of 96 samples within a short time, achieving recoveries of more than 95% for various samples. When using column-based technologies, processing multiple samples in academic research labs may necessitate a significant quantity of hand pipetting. This pipetting can discourage differences in the yield of target biomolecules between experiments and people. Staff and students may require extensive training and practice to produce constant protein yields. It is due to the numerous benefits of magnetic resins, such as their ease of use, rapid experimental protocols, suitability, and convenience for high throughput automated and miniaturized processing.
●
Excellent recovery free of reducing agent—Removing the reducing agent and recovering the reduced molecule without sample loss is a difficulty inherent in DTT or -mercaptoethanol (BME). Immobilized TCEP enables you to recover protein/peptides in a high yield (90% or higher) without dialyzing or desalting.
●
Odorless — Unlike DTT or BME, Immobilized TCEP is odorless, allowing reductions to be performed on the bench top.
●
Air stability inherent TCEP stability eliminates the need for particular measures to avoid oxidation when handling, using, or storing Immobilized TCEP Disulfide Reducing Beads.
●
Simple to use — The immobilized TCEP reductant resin allows you to dispense the quantity of support needed for each application. Reductions can be carried out at various pH levels (from 4 to 9) and temperatures (5 to 95°C).
Note
●
Reduction occurs over a wide pH (pH 4.0-9.0) and temperature range (5°-95°C).
●
Most proteins can be effectively reduced without the use of a denaturant. However, adding a denaturant such as 6M guanidine•HCl will help expose interior disulfides to the immobilized TCEP and assure complete reduction. Urea is not suggested as a denaturant because it generates cyanates that react with sulfhydryl groups.
●
●
Because disulfides regenerate over time, the reduced sample should be used promptly after reduction.
●
TCEP-immobilized beads are intended for one-time usage only.
The following protocol is an example of Disulfide Reduction. To get the best results, we recommend performing a titration to optimize the amount of beads used for each application. The protocol can be scaled up/down.
Buffer
●
20 mM EDTA
●
Equipment
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Item
Adjustable Single and Multichannel Pipettes
Item
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR Plates/Tubes
** IMPORTANT! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Adjustable Single and Multichannel Pipettes
Procedure
! Important !
The following protocol is an example of Disulfide Reduction. To get the best results, we recommend performing a titration to optimize the amount of beads used for each application. The protocol can be scaled up/down.
A. Magnetic Particles Preparation
1.
Suspend the desired amount of the Bead with dH2O at a concentration of 50 mg/ml.
Note: For the best results, prepare fresh beads. The suspended beads can be stored at 4ºC for one week without reducing activity.
2.
Shake or vortex the bottle to completely resuspend the magnetic beads before using.
Note: Do not allow the magnetic beads to sit for more than two minutes before dispensing.
B. Sample preparation
1.
Transfer desired amount of magnetic particles to a centrifuge tube.
Note: Optimize the amount of beads used for each application. Typically, 30-50μl (3mg-5mg beads) of TCEP disulfide reducing magnetic beads for a 30-50 μL protein/peptide sample (50-80 μg).
2.
Place the tube on the magnetic rack for 1-3 minutes until the supernatant becomes clear. Remove the supernatant while the tube remains on the rack.
3.
Add the samples and completely mix by slowly pipetting up and down 25 times (one minute) or by vortex mixer for 5 minutes at 2000 rpm.
4.
Leave them at room temperature for 1 hour with end-over-end rotation.
5.
Place the tube on a magnetic rack for 1-3 minutes. Transfer the supernatant to a fresh tube while the tube remains on the rack.
Troubleshooting
Problem
Poor reduction of sample
Probable Cause
Less amount of beads used
Suggestion
Use the recommended amount of beads
Problem
Poor reduction of sample
Probable Cause
Incubation time is too short.
Suggestion
Increase incubation time
Problem
Poor reduction of sample
Probable Cause
Disulfides were sterically inaccessible in protein.
Suggestion
Add 6 M guanidine•HCl to the reduction buffer.
Problem
Poor reduction of sample
Probable Cause
Incubation time is too long.
Suggestion
Do not exceed a 2-hour incubation
Problem
Loss of reducing the capacity of beads
Probable Cause
The product was stored for more than one-year-old.
Suggestion
Purchase new product
Problem
Probable Cause
Suggestion
Poor reduction of sample
Less amount of beads used
Use the recommended amount of beads
Incubation time is too short.
Increase incubation time
Disulfides were sterically inaccessible in protein.
Add 6 M guanidine•HCl to the reduction buffer.
Incubation time is too long.
Do not exceed a 2-hour incubation
Loss of reducing the capacity of beads
The product was stored for more than one-year-old.
Purchase new product
1.
Zwyssig A, Schneider EM, Zeltner M, Rebmann B, Zlateski V, Grass RN, Stark WJ. Protein Reduction and Dialysis-Free Work-Up through Phosphines Immobilized on a Magnetic Support: TCEP-Functionalized Carbon-Coated Cobalt Nanoparticles. Chemistry. 2017 Jun 27;23(36):8585-8589.
2.
Tzanavaras PD, Mitani C, Anthemidis A, Themelis DG. On-line cleavage of disulfide bonds by soluble and immobilized tris-(2-carboxyethyl)phosphine using sequential injection analysis. Talanta. 2012 Jul 15;96:21-5.
3.
Han J, Clark C, Han G, Chu TC, Han P. Preparation of 2-nitro-5-thiobenzoic acid using immobilized Tris(2-carboxyethyl)phosphine. Anal Biochem. 1999 Mar 15;268(2):404-7.
4.
Han, J.C., et al. A procedure for quantitative determination of tris(2-carboxyethyl)phosphine, an odorless reducing agent more stable and effective than dithiothreitol. Anal Biochem (1994) 220:5-10
Magnetic Beads Make Things Simple