- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
Fluorophore
Excitation
(nm)
Emission
(nm)
Fluorescence lifetime
(Ԏ) (µsec)
Stokes shifts
(nm)
Ruthenium (Ru2+)
Far-Red
470
710
354.36
175
Fluorescence Dye Properties
Fluorophore
Ruthenium (Ru2+)
Far-Red
470
710
Fluorescence lifetime (Ԏ) (µsec)
354.36
Stokes shifts
(nm)
175
Specification
Bead Size
2.5μm diameter; 5μm diameter
Number of Beads
~ 1.47 x 108 beads/mg (2.5μm beads)
~ 5 x 107 beads /mg (5μm beads)
Stability
Short Term (<1 hour): pH 3-11; Long-Term: pH 4-10
Temperature: 4°C -140°C; Most organic solvents
Magnetization
~ 40-45 EMU/g
Type of Magnetization
Superparamagnetic
Formulation
Lyophilized Powder
Functional Group Density
2.5μm Magnetic Beads
~ 240 μmole / g of Beads
5μm Magnetic Beads
~ 200 μmole / g of Beads
Storage
Store at -20°C upon receipt.
BcMag™ Aldehyde-activated Ruthenium Fluorescence Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres Time-Resolved Fluorescence (TRF) magnetic microspheres grafted with a high density of aldehyde functional groups on the surface. The bead aldehyde groups react spontaneously with primary amines present at the N-terminus of proteins or in lysine residues to form intermediate Schiff Base complexes. The reaction of reductive amination immobilization starts with the creation of an initial Schiff base between the aldehyde and amine groups, which is then reduced to a secondary amine by adding sodium cyanoborohydride (NaCNBH3) to generate stable amine bonds between the bead and the ligand. At physiological to alkaline circumstances (pH 7.2 to 9) in either aqueous or organic solvents with 20% ~ 30% DMSO or DMF, coupling reaction takes 2 to 6 hours in a one-step process. Coupling efficiency with antibodies and normal proteins is usually better than 85%, resulting in 15 – 20 μg /mg of beads.
The microspheres combine the benefits of the active aldehyde group, time-resolved Fluorescence dyes, and magnetic characteristics to perform very sensitive assays. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and ruthenium metal as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and ruthenium metal. The hydrophilic surface provides beads with low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
Although conventional fluorophores have been widely used over the past decades, they still suffer from either one or several limitations in terms of applicability and efficiency:
1.
Narrow excitation bands cause higher background signals.
2.
Smaller Stokes shift often produces self-quenching.
3.
Fluorescence is sensitive to environmental factors such as metallic ion concentration, pH, temperature, and solvent polarity.
4.
Fluorescence intensity is not high enough for detecting a single biomolecular.
5.
Fluorescence intermittency (blinking) affects some processes of molecule detection.
6.
Easily aggregated because of hydrophobicity.
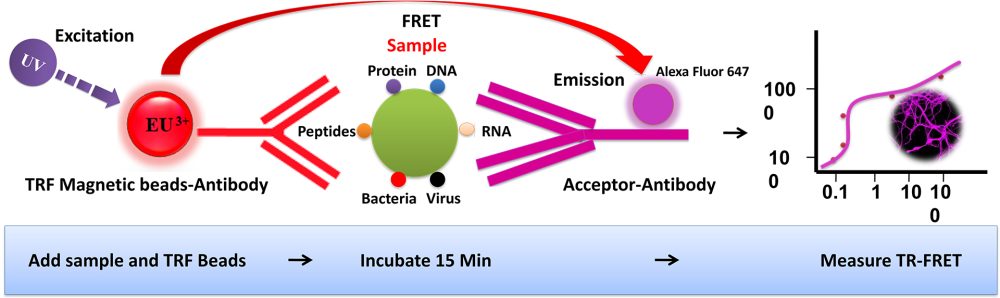
BcMag™ TR-FRET Assay, in contrast to typical FRET (Förster Resonance Energy Transfer) assays, uses time-resolved Fluorescence magnetic beads (BcMag™ TR-Magnetic Beads) as the donor fluorophore. The donor and acceptor can be two proteins, two DNA strands, an antigen, an antibody, or a ligand and its receptor. After a reasonable time delay (usually 50 to 100 s), a signal is generated by fluorescence resonance energy transfer between a donor and an acceptor molecule when they are close and monitored in a time-resolved way. In BcMag™ TR-FRET Assay, a trace amount of analytes can be easily enriched from the complex by TR-Magnetic Beads, resulting in higher sensitivity. This assay practically eliminates all fluorescence backgrounds caused by the sample and plastic microplate, as well as by direct acceptor excitation. As a result, the signal-to-noise ratios of the BcMag™ TR-FRET Assay are very high, and the background is quite low. Furthermore, the assay does not need washing steps. BcMag™ TR-FRET Assay offers substantial advantages to bioassays in high throughput screening, such as assay flexibility, dependability, increased assay sensitivity, higher throughput, and fewer false positive/false negative results.
Ruthenium cryptate fluorophore is an efficient time-resolved Fluorescence tag due to its distinct specific properties. It is excited at 470nm and emits red fluorescence at 710nm, with a long fluorescence lifetime (354.36 μsec) and large stokes shifts (175 nm). BcMag™ Amine-activated Ruthenium Fluorescence Magnetic Beads are available in nominal diameters of 2.5μm and 5μm. By taking advantage of these properties, time-resolved fluorescence measurement can dramatically reduce the fluorescence background from the sample and increase the signal-to-noise ratio to offer detectability better than one order of magnitude than conventional Fluorescence dyes. BcMag™ Amine-activated Ruthenium Fluorescence Magnetic Beads are excellent donors used in TR-FRET assays.
1.
Mix the antibody-conjugated donor beads with the cell lysates and incubate them with continuous rotation for a sufficient time. The beads remain suspended in the sample solution during mixing, allowing the target analytes to bind to the donor beads.
2.
After incubation, the beads are collected and separated from the sample using a magnet rack.
3.
Add the antibody-conjugated acceptor and incubate them with continuous rotation for a sufficient time.
4.
Analysis of numerous microplate readers supports TR-FRET measurements.
1.
Perform a double function simultaneously on the same beads: The magnetic beads combine separation/preconcentration and detect analytes, allowing quick, simple, robust, and high-throughput analytes of trace amounts from complex biological samples on the same beads.
2.
Ultra sensitive. Lower detection limits of 10 pg/mL versus typical fluorometric detection limits of 100 pg/mL.
3.
Extremely photostable and highly resistant to photobleaching. All the lanthanide chelate or cryptate molecules and iron oxide are entirely encapsulated inside each bead instead of merely on the bead’s surface. The protective environment prevents iron oxide and dye from leaching into aqueous media, which makes the beads less sensitive to external conditions such as solvent, temperature, pH, etc.
4.
Very high Fluorescence intensity. Because a single bead has a large concentration of lanthanide chelate with a high quantum yield ranging from 40 to 90%, the beads show excellent fluorescence intensity, which increases test sensitivity without signal amplification. Such bright beads are also perfect for donors’ use in time-resolved FRET assays.
5.
Lanthanide chelate or cryptate has large Stokes shifts (>250 nm), narrow emission bands (-10 nm bandwidth), and long fluorescence lifetime (μs), which dramatically reduces background and increases the signal-to-noise ratio.
6.
Most bioprocess ELISA assays can be converted to an HTRF assay.
7.
No washing step is involved in the assays.
8.
Have a hydrophilic silica surface grafted by different functional groups with linkers of variable lengths, allowing efficient conjugation of various ligands such as peptides, proteins, antibodies, small molecules, carbohydrates, aptamers, DNA/RNA, etc.
9.
Due to the microsphere’s magnetic properties, the Fluorescence magnetic beads are suitable for high-throughput automation.
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.
Magnetic Beads Make Things Simple