- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Kit Components
CA101
5 ml
Carboxy-terminated Magnetic beads
5 ml
2x Suspension Buffer
10 ml
10x Washing Buffer
0.15 g
CA102
10 ml
Carboxy-terminated Magnetic beads
10 ml
2x Suspension Buffer
20 ml
10x Washing Buffer
0.3 g
Specificities
Composition
Carboxy-terminated magnetic beads
Bead Size
1µm diameter
Number of Beads
~1.7 x 108 beads/mg
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.5 g/ml
Stability
pH 4-10
Concentration
Binding Capacity
>10 µg Oligo-DNA (25 nucleotides)/mg
Storage
Store at 4°C upon receipt
The BcMag™ Quick Oligo-DNA Conjugation Kit is a powerful tool designed for researchers who need to quickly and efficiently immobilize oligonucleotide DNA to proprietary magnetic beads. The kit uses cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) to covalently immobilize amine-modified oligonucleotides to the surface of carboxyl-activated magnetic beads, resulting in a more secure attachment compared to other methods.
The workflow for using the BcMag™ Quick Oligo-DNA Conjugation Kit is simple and straightforward. First, the carboxyl-activated magnetic beads are washed and resuspended in a buffer. The amine-modified oligonucleotide is then added to the magnetic bead solution, along with the EDC cross-linker. The reaction is allowed to proceed overnight, during which time the oligonucleotide is covalently immobilized to the magnetic bead surface.
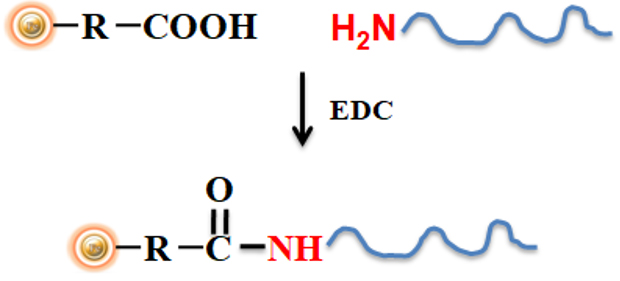
The resulting magnetic bead-oligonucleotide conjugate is then washed and resuspended in buffer, ready for use in downstream applications such as PCR, hybridization, and sequencing.
●
Quick, Easy, and one-step protocol
●
Neutral linkage – Forms neutral amide bonds between carboxyl and amines
●
Stable covalent bond with minimal ligand leakage
●
High immobilization efficiency
●
Scalable – Easily adjusts for sample size and automation
●
Reproducible results
The BcMag™ Quick Oligo-DNA Conjugation Kit is ideal for a wide range of applications, including PCR, hybridization, and sequencing. The resulting magnetic bead-oligonucleotide conjugate can be used for a variety of downstream applications, including RNA and DNA sequencing, mutation detection, and genotyping.
The protocol can be scaled appropriately up or down.
Materials Required
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following magnetic Racks:
For larger scale purification, Ceramic magnets Block for large scale purification (6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8)
●
Corning 430825 cell culture flask for large-scale purification (Cole-Parmer, Cat. No. EW-01936-22)
●
Mini BlotBoy 3D Rocker, fixed speed, small 10″ x 7.5″ platform w/ flat mat (Benchmark Scientific, Inc. Cat. No. B3D1008) or compatible
A. Oligo-DNA Preparation
1.
The oligo-DNA must be modified with an amino group at either 5’or 3′ end (A commercial oligo synthesis company can provide such service).
Note:
It is strongly recommended that 10-15 extra nucleotides be added to your oligo DNA sequence between the terminal amino group and your DNA sequence to overcome steric hindrance when you design your target oligo–DNA sequence. The oligo-DNA should be purified by standard desalting or other methods.
2.
Resuspend the oligo-DNA in 1x suspension buffer at concentrations of 5-10 µg/ul (Optional: Aspirate 20 µl from the oligo solution, transfer to a new centrifuge tube, and label the tube as C1)
B. Coupling Buffer Preparation
1.
Prepare coupling buffer by adding 19 mg EDC to 1ml of 1x suspension buffer (Coupling buffer must be prepared fresh immediately before use)
C. Coupling
1.
Shake the bottle to resuspend the BcMag Magnetic Beads thoroughly.
2.
Transfer 1ml magnetic beads (20 mg/ml) to a tube. Place the tube on the magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack.
3.
Remove the tube from the Rack and resuspend the beads thoroughly with 1ml suspension buffer. Place the tube on the magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack.
4.
Repeat step 3 once.
5.
Remove the tube from the Rack and resuspend the beads thoroughly with a 200µl coupling buffer. Mix the magnetic beads with 100-200 µg oligo-DNA prepared from Step A.2
6.
Incubate the beads overnight at 50° C with continuous rotation.
7.
Place the tube on a magnetic Rack for 1-3 minutes. Remove the supernatant while the tube remains on the Rack (Optional: Aspirate 20 µl supernatant, transfer to a new centrifuge tube, and label the tube as C2).
8.
Wash the beads three times with 1 ml of washing buffer at room temperature and twice with dH20 at 65° C.
9.
Resuspend the beads at 5 mg/ml in PBS buffer containing 0.2% NaN3 and store them at 4° C.
Coupling Efficiency Calculation
1.
Measure OD at A260
Coupling Efficiency (%) = [(C1-C2)/C1] x 100%
C1: A260 Pre-Coupling oligo DNA Solution x dilution factor; C2: A260 post-Coupling oligo DNA Solution
2.
Using fluorescent dye to quantify C1 and C2.
1.
Ferrier DC, Shaver MP, Hands PJW. Micro- and nano-structure-based oligonucleotide sensors. Biosens Bioelectron. 2015 Jun 15;68:798-810.
2.
Sethi D, Gandhi RP, Kuma P, Gupta KC. Chemical strategies for immobilization of oligonucleotides. Biotechnol J. 2009 Nov;4(11):1513-29.
3.
Zuo P, Ye BC. A novel immobilization strategy using oligonucleotide as linker for small molecule microarrays construction. Biosens Bioelectron. 2008 Jun 15;23(11):1694-700.
Magnetic Beads Make Things Simple