- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Components
Shipping conditions: At ambient temperature
Components
BcMag™ U-DNA Beads
10x Lysis Buffer (100mM Tris-HCl, PH 9.0)
Proteinase K
DTT
Proteinase K Suspension Buffer
Storage
4°C
4°C
-20°C
-20°C
4°C
Cat. No. AS101 (50 Preps)
2.5 ml
0.6 ml
12.5 mg
15.4 mg
1.0 ml
Cat. No. AS102 (100 Preps)
5.0 ml
1.2 ml
25 mg
30.8 mg
2.0 ml
Shipping conditions: At ambient temperature
Handling and Storage: Store the kit components according to the table Above on arrival.
Touch DNA is defined as DNA collected from lost skin cells and other biological material left on touched surfaces by the papillary ridge patterns found on fingers, palms, toes, and soles. This evidence is extremely helpful in many criminal cases, from theft to sexual violence to murder. For example, in cases of murder or sexual assault, precious evidence could be gathered by studying the steering wheel of a vehicle used in thefts or weapons and clothing in cases of murder or sexual assault. Furthermore, collecting “Touch DNA” from a crime scene to identify a person of interest could be very valuable, especially in the absence of other types of biological evidence.
Typically, the DNA profiling process begins with the extraction of DNA from the substrate. DNA collection and recovery procedure remains the most critical in the DNA analysis process. Almost all experiments require an adequate amount of quantitative and qualitative DNA. As a result, the success of DNA typing is dependent on the availability of existing DNA templates. Better methods for recovering DNA are required, especially with touch samples containing a tiny amount of DNA. Various approaches have been utilized to increase evidential DNA’s quantity and quality. However, depending on the extraction method and the quantification method’s accuracy, the DNA extraction procedure can result in a loss of 20% to 90% of the initial template amount. The current purification technique utilized in forensic DNA cases is time-consuming and labor-intensive.
Furthermore, the column-based purification procedures result in DNA loss, preventing the successful typing of low copies or damaged samples. Direct PCR amplification has been proposed as one of the approaches for avoiding DNA loss from touch evidence samples. By skipping the extraction, quantification, and concentration steps, the majority of DNA can be retained. Laboratory employee error and DNA contamination from handling can be eliminated, and overall sample processing time and cost can be decreased. Although many DNA samples are extracted directly from the solid support, such as clothing, upholstery, paper, chewing gum, and cigarette butts, direct extraction can carry high concentrations of PCR inhibitors. Bioclone developed a revolutionary one-step DNA purification system based on magnetic beads to overcome these drawbacks.
BcMag™ One-Step Touch DNA Purification Kit uses novel Negative chromatography magnetic beads to quickly deliver higher quality and superior DNA yield from most trace touch samples. Those samples include body fluids, stains, swabs of body fluids, Strip removed cells, cigarette butts, Hair follicles, fingernail scrapings, epithelial cells, bite marks, semen, touch DNA samples, etc. The specially designed magnetic beads with our proprietary surface chemistry function simultaneously to lyse cells and capture the PCR inhibitors (Fig.1) once mixed with the sample.
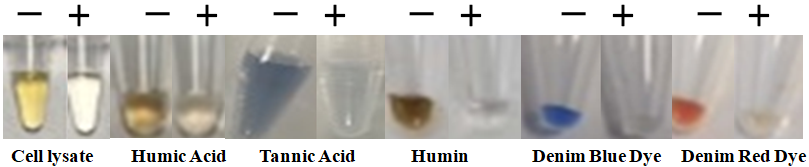
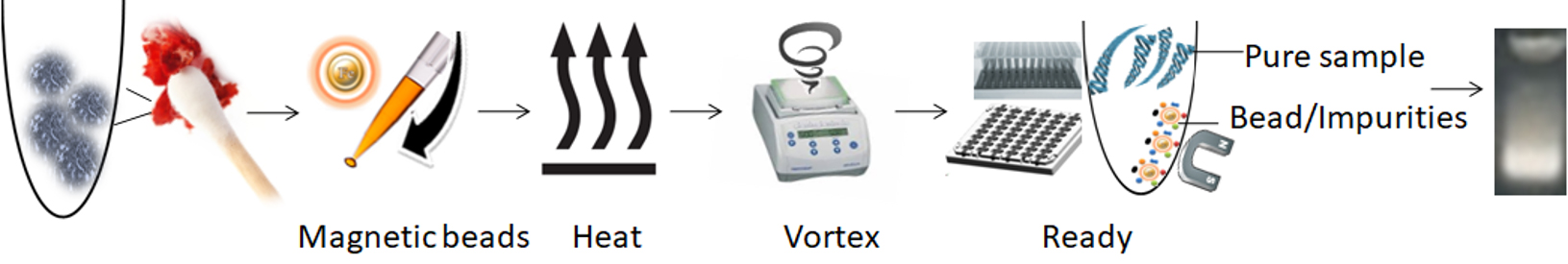
The magnetic beads-PCR inhibitor complex was then magnetically removed by a magnet while the pure DNA remaining in the solution was ready for downstream STR analysis. The purification kit provides a fast and simple method for DNA purification with only one tube, no liquid transfer, and no requirement for carrier RNA. It reduces the risk of DNA loss and carryover of extraction buffers from the traditional and tedious bind-wash-elute procedure. After preparing the lysates, it enables the processing of 96 samples in less than 15 minutes, with less than 1 minute of hands-on Time.
1.
Add functional magnetic beads to the sample.
2.
Mix the samples with the magnetic beads and proteinase K to lyse the cells.
3.
Mix by vortexing/pipetting for the beads to capture the PCR inhibitors.
4.
Remove the beads with a magnet.
5.
Aspirate the supernatant containing the pure ready-to-use DNA
The purified DNA is suitable for use in sensitive downstream applications, such as PCR, qPCR, single-nucleotide polymorphism (SNP), short tandem repeat (STR) genotyping, genotyping, or next-generation sequencing (NGS).
●
Rapid and efficient purification protocol: without prior DNA isolation for subsequent use in direct workflows, No liquid transfer, and One-tube.
●
Ultrafast: Process 96 samples in less than an hour.
●
Highest nucleic acids recovery rates: Minimal loss of DNA during extraction
●
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High-throughput: Compatible with many different automated liquid handling systems.
This product is intended for forensic, human identification, and paternity testing molecular biology applications. This product is not designed for disease diagnosis, prevention, or treatment.
1.
Van Oorschot, R., Jones, M. DNA fingerprints from fingerprints. Nature 387, 767 (1997).
2.
Tang J, Ostrander J, Wickenheiser R, Hall A. Touch DNA in forensic science: The use of laboratory-created eccrine fingerprints to quantify DNA loss. Forensic Sci Int Synerg. 2019 Oct 23;2:1-16.
3.
Jo-Anne Bright, Susan F. Petricevic. Recovery of trace DNA and its application to DNA profiling of shoe insoles, Forensic Science International, Volume 145, Issue 1, 2004, Pages 7-12
4.
Tang J, Ostrander J, Wickenheiser R, Hall A. Touch DNA in forensic science: The use of laboratory-created eccrine fingerprints to quantify DNA loss. Forensic Sci Int Synerg. 2019 Oct 23;2:1-16.
Magnetic Beads Make Things Simple