- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Specification
Composition
Magnetic beads are modified with our proprietary chemistry.
Stability
Short Term (<1 hour): pH 4-11; Long-Term: pH 4-10
Temperature: 4°C -140°C; Most organic solvents
Magnetization
~40-45 EMU/g
Type of Magnetization
Superparamagnetic
Formulation
Binding Capacity
50 µg proteins/mg of Beads
Storage
Ship at room temperature, Store at 4°C upon receipt.
The presence of protein and lipids in biological fluid samples frequently interferes with small molecule analysis, decreasing accuracy and sensitivity. Numerous bioassays need the removal of proteins (deproteinization) and lipids (delipidation) from samples before analysis, such as LC/MS/MS analysis.
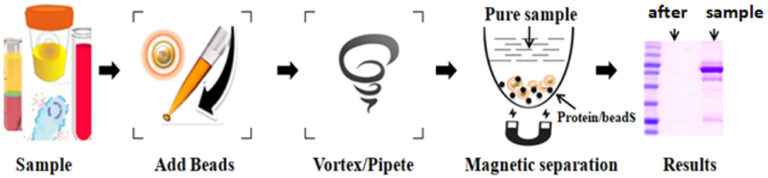
Precipitation using trichloroacetic acid, perchloric acid, phosphotungstic acid, acetonitrile, methanol, and other organic solvents has been extensively used for deproteinization over the last half-century. However, these procedures are not only tedious, time-consuming, and challenging to adapt to automation but also inefficiently remove lipids and nucleic acids. BcMag™ One-Step Deproteinizing Kit provides a novel solution for the deproteinization, delipidation, and nucleic acid from biological fluid samples such as cell lysis, plasma, serum, tissue homogenate, and urine for analysis of a variety of small molecules. The superparamagnetic magnetic beads efficiently utilize the proprietary matrix to bind proteins and lipids in the sample. The one-minute protein removal protocol is straightforward: one step, one tube, no precipitation, no filtration, and no centrifugation. The resin enables 96 samples to be processed simultaneously in less than 10 minutes.
●
Fast and straightforward protocol: one step, one tube, process 96 samples in less than 10 minutes
●
Easy-to-use: Eliminates columns, filters, laborious repeat pipetting or centrifugation, and easily adjusts for sample size and automation
●
No organic solvents/acids precipitation
●
Efficient deproteinization
A. Materials Required by the User
Item
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
• BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
• BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
• BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
• BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
Item
BcMag™ 96-well Plate Magnetic Rack.
Source
• BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Item
Adjustable Single and Multichannel Pipettes
Item
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates / tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Eppendorf, Cat. No. 5353000529
Tube Holder PCR 96
Eppendorf, Cat. No. 022674005
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 022674048
Smart Mixer, Multi Shaker
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR plates/tubes
** IMPORTANT! If using other tubes or PCR plates, make sure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
Items
Magnetic Rack for centrifuge tube
** Based on sample volume, the user can choose one of the following magnetic Racks
Source
●
BcMag™ Rack-2 for holding two individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-01)
●
BcMag™ Rack-6 for holding six individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-02)
●
BcMag™ Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Bioclone, Cat. No. MS-03)
●
BcMag™ Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Bioclone, Cat. No. MS-04)
BcMag™ 96-well Plate Magnetic Rack
●
BcMa™ 96-well Plate Magnetic Rack (side-pull) compatible with 96-well PCR plate and 96-well microplate or other compatible Racks (Bioclone, Cat. No. MS-05)
Adjustable Single and Multichannel Pipettes
Centrifuge with Swinging Bucket
Addition items are required if using 96-well PCR plates/tubes
Vortex Mixer
** The user can also use other compatible vortex mixers. However, the Time and Speed should be optimized, and the mixer should be: Orbit ≥1.5 mm-4 mm, Speed ≥ 2000 rpm
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Smart Mixer, Multi Shaker
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
Eppendorf, Cat. No. 022674048
BenchTop Lab Systems, Cat. No. 5353000529
Eppendorf™ MixMate™
Tube Holder PCR 96
Tube Holder 1.5/2.0 mL, for 24 × 1.5 mL or 2.0 mL
Eppendorf, Cat. No. 5353000529
Eppendorf, Cat. No. 022674005
BenchTop Lab Systems, Cat. No. 5353000529
1.5/2.0 mL centrifuge tube
96-well PCR Plates or 8-Strip PCR Tubes
PCR Plates/Tubes
! IMPORTANT ! If using other tubes or PCR plates, ensure that the well diameter at the bottom of the conical section of PCR Tubes or PCR plates must be ≥2.5mm.
B. Procedure
Notes:
●
Do not use buffers containing organic solvents.
●
For the best results, ensure that the sample pH should be ~4.5.
1.
Dilute and adjust the protein concentration in sample ~1mg/ml and pH to 4.5 by sodium acetate or other buffers.
2.
Shake the bottle to resuspend the Magnetic Beads until it is homogeneous completely.
! IMPORTANT !
3.
Add an appropriate amount of the magnetic Beads to the sample (Beads amount should be calculated based on the sample protein concentration and the Beads binding capacity, ~50µg protein/mg Beads.)
4.
! IMPORTANT !
5.
Place the sample plate or tube on the magnetic rack for 30 seconds or until the solution is clear.
(Option: centrifuge at 3500 rpm for 45 seconds)
6.
Transfer the supernatant to a clean plate/tube while the sample plate remains on the magnetic separation plate. The sample is ready for downstream applications.
C. Troubleshooting
Problem
Deproteinization efficiency is low.
Probable Cause
Suggestion
Problem
Probable Cause
Suggestion
Deproteinization efficiency is low.
1.
Hušek P, Svagera Z, Hanzlíková D, Simek P. Survey of several methods deproteinizing human plasma before and within the chloroformate-mediated treatment of amino/carboxylic acids quantitated by gas chromatography. J Pharm Biomed Anal. 2012 Aug-Sep;67-68:159-62. doi: 10.1016/j.jpba.2012.04.027. Epub 2012 May 7. PMID: 22633606.
2.
Sakuma R, Nishina T, Kitamura M. Deproteinizing methods evaluated for determination of uric acid in serum by reversed-phase liquid chromatography with ultraviolet detection. Clin Chem. 1987 Aug;33(8):1427-30. PMID: 3608161.
3.
Álvarez-Guerra ED, Parkes HG, Bell JD. Comparative study of two deproteinization methods for the evaluation of whole blood and plasma by magnetic resonance spectroscopy. Bioquimia. 2006;31(2):59-68.
4.
Daykin CA, Foxall PJ, Connor SC, Lindon JC, Nicholson JK. The comparison of plasma deproteinization methods for the detection of low-molecular-weight metabolites by (1)H nuclear magnetic resonance spectroscopy. Anal Biochem. 2002 May 15;304(2):220-30. doi: 10.1006/abio.2002.5637. PMID: 12009699.
5.
Chen, Y., Xie, M., Li, W. et al. An effective method for deproteinization of bioactive polysaccharides extracted from lingzhi (Ganoderma atrum). Food Sci Biotechnol 21, 191–198 (2012).
6.
Magnetic Beads Make Things Simple