- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Recombinant protein is a genetically engineered protein encoded by recombinant DNA. The recombinant DNA has been cloned in a system allowing gene expression and messenger RNA translation. Protein synthesis using recombinant DNA technology has been one of the most groundbreaking discoveries in scientific research in recent years. Previously, the only technique to make proteins was to isolate them from their natural source. Nowadays, DNA sequences encoding the desired protein can be cloned into a vector and introduced into an expression system, such as mammalian cells, bacteria, yeast, or insect cells, where it can be easily expressed and purified. Such new technology has enabled some of the most revolutionary and forward-thinking discoveries in proteomics research. Recombinant proteins are widely used in the production of pharmaceuticals, protein-based polymers for drug administration, antibodies and enzymes for disease therapy, protein scaffolds for tissue engineering, and various other applications.
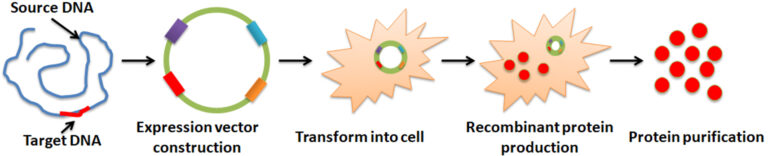
Recombinant protein production is a biotechnological process involving synthesizing a specific protein. It is often accomplished by manipulating gene expression in an organism so that a recombinant gene is expressed in large quantities. The process comprises: 1. gene amplification of interest by Polymerase chain reaction (PCR). 2. Incorporate the amplified gene into the expression vector for the following replication and protein expression. 3. Introduction of the vector into a host cell, such as bacteria, yeast, mammalian cells, or the baculovirus-insect cell system, where the cell’s protein synthesis machinery creates the desired protein. 4. Large-scale production and purification.
Polymerase chain reaction (PCR) is an amplification technique that makes thousands to millions of copies of DNA of interest by cloning specific or targeted sections of a DNA sequence. After the PCR reaction, it is necessary to purify the product from the rest of the PCR reaction, ligate it into a suitable expression vector, and sequence it to confirm the DNA sequence of interest.
Explore
Transfecting a specific cell with a DNA vector containing the recombinant DNA template is the approach for producing recombinant protein. The cells bearing the template are then grown for them to transcribe and translate the desired protein. These cells are subsequently lyzed or broken to release the produced protein, purified using various processes. Both prokaryotic and eukaryotic systems have been employed to express the recombinant DNA. This decision is frequently made depending on the protein type, functional activity, and necessary yield.
Bacterial System
Bacterial protein expression systems are popular because bacteria are simple to culture, grow quickly, and create large amounts of recombinant protein. However, multi-domain eukaryotic proteins produced in bacteria are frequently non-functional because the cells lack the necessary posttranslational modifications or molecular folding. Furthermore, many proteins become insoluble as inclusion bodies, which are extremely difficult to recover without strong denaturants and time-consuming protein-refolding methods.
A mammalian host system is the optimum expression platform for creating mammalian proteins with the most native structure and activity. Because it provides for the maximum level of posttranslational processing and functional activity of the protein, mammalian expression is the system of choice for researching the function of a specific protein in the most physiologically relevant environment.
Insect System
This system performs functional experiments, structural analysis, intracellular protein and protein complex expression, virus generation, and so on. The methods used to process proteins in this situation are similar to those used in mammalian systems. They are commonly employed to produce antibodies, therapeutic proteins, and proteins for human usage in functional cell-based tests. However, the cell culture conditions in this system are more demanding than those in prokaryotic cells. In addition, generating recombinant vectors in insect systems takes time.
Yeast System
This system is utilized for structural analysis, functional assays, antibody production, and protein interaction research. This technique is scalable up to fermentation and can be used to process eukaryotic proteins. However, fermentation is essential for high yields, and growth conditions must frequently be optimized. In this situation, the media requirements are likewise straightforward.
Cell-Free System
This approach investigates hazardous proteins, the integration of unnatural amino acids, and the screening of translational inhibitors, among other things. Because this is an open system, it can add artificial components. Furthermore, the statement is simple. Scaling up recombinant protein production beyond multi-milligram volumes, on the other hand, may be costly and time-consuming.
How to purify the recombinant protein?
Protein purification can be difficult and may necessitate a combination of different chromatography procedures to achieve good results. When building a purifying operation, it is critical to keep downstream applications in mind. A high-purity, properly-folded protein is required for applications such as crystallography. In other circumstances, functional purity is sufficient; if a protein of interest is assayed for an activity that can be properly identified even in the presence of low-level impurities, it may not be required to perform additional purification processes to remove these contaminants.
The most common approach for protein purification is affinity chromatography, which separates proteins based on their unique interaction with a matrix. It is one of the most effective approaches because it uses the insertion of the desired structure (called a tag, such as His-tag, GST-tag) onto the protein. This tag is not found in any other molecule in the sample, giving our target protein special features that will be used to recognize and separate it from the others. However, in some cases, we cannot add a tag to our molecule and must resort to less specific ways (although they can be equally effective if they are used correctly).
Explore
Gel filtration is one of these methods, which separates molecules based on their size. Molecules can be sorted by their difficulty when passing through a resin with holes of a given diameter. Ion exchange chromatography is another widely used technology that separates molecules based on their electric charge under specific pH and temperature conditions. Hydrophobic interaction chromatography and reverse-phase chromatography are the most often used methods for separating proteins depending on their polarity. The critical distinction between these approaches is the polarity of the matrix with which the purified protein interacts.
Explore
Recombinant proteins are extensively utilized in the production of pharmaceuticals, protein-based polymers for drug administration, antibodies and enzymes for disease therapy, protein scaffolds for tissue engineering, and various other applications.
Recombinant proteins now account for the vast majority of top-selling medications, including those used to treat complicated disorders ranging from arthritis to cancer, and those used to battle infectious diseases such as COVID-19 by neutralizing antibodies.
Recombinant pathogenic protein-based serological assays may attain excellent sensitivity and specificity because of the high concentration of immunoreactive antigens and the lack of nonspecific moieties present in whole-cell preparations from the blood of experimentally infected animals. A vaccinated person creates antibodies against the protein antigen, which protects them from acquiring the disease when the pathogenic microorganism attacks.
Recombinant protein vaccines have a long history in the industry, dating back to the mid-1980s with the hepatitis B vaccine, which is now a common vaccination worldwide. These vaccines represented the first step away from traditional manufacturing, overcoming numerous difficulties in vaccine development and manufacture. Unlike antigen purification from an inactivated pathogen, recombinant antigen synthesis allows for high expression levels and increases vaccination safety.
Explore
1.
Imataka H, Mikami S (2009) Advantages of human cell-derived cell-free protein synthesis systems. Seikagaku 81(4):303–7.
2.
Mikami S et al. (2008) A human cell-derived in vitro coupled transcription/translation system optimized for production of recombinant proteins. Protein Expr Purif 62(2):190–8.
3.
Kobayashi T et al. (2007) An improved cell-free system for picornavirus synthesis. J Virol Methods 142(1-2):182–8.
4.
5.
6.
Oliveira C, et al. (2018) Guidelines to reach high-quality purified recombinant proteins. Appl Microbiol Biotechnol 102(1): 81-92.
7.
Kosobokova EN, et al. (2016) Overview of fusion tags for recombinant proteins. Biochemistry (Mosc) 81(3): 187-200.
8.
Wingfield PT (2015) Overview of the purification of recombinant proteins. Curr Protoc Protein Sci 80: 6.1.1-6.1.35.
9.
Assenberg R, et al. (2013) Advances in recombinant protein expression for use in pharmaceutical research. Curr Opin Struct Biol 23(3): 393-402.
10.
Ohba Y, et al. (2013) Fluorescent protein-based biosensors and their clinical applications. Prog Mol Biol Transl Sci 113: 313-348.
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.