- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

A recombinant protein is a genetically engineered protein encoded by recombinant DNA. The recombinant DNA has been cloned in a system allowing gene expression and messenger RNA translation. Protein synthesis using recombinant DNA technology has been one of the most groundbreaking discoveries in scientific research in recent years. Previously, the only technique to make proteins was to isolate them from their natural source. Nowadays, DNA sequences encoding the desired protein can be cloned into a vector and introduced into an expression system, such as mammalian cells, bacteria, yeast, or insect cells, where it can be easily expressed and purified. Such new technology has enabled some of the most revolutionary and forward-thinking discoveries in proteomics research. Recombinant proteins are widely used in the production of pharmaceuticals, protein-based polymers for drug administration, antibodies and enzymes for disease therapy, protein scaffolds for tissue engineering, and various other applications.
Recombinant protein purification is the process of isolating and purifying a specific protein that has been produced through genetic engineering techniques. This typically involves several steps, including cell lysis, centrifugation, and chromatography. The first step is to break open the host cells in which the protein is expressed, a process called cell lysis. This can be done by mechanical means, such as grinding or sonication, or by chemical means, such as using detergents or enzymes. Next, the lysate is centrifuged to remove cellular debris and other impurities. Depending on the specific protein and the host organism, it may be necessary to perform additional steps to further purify the protein. Chromatography is the most commonly used method for purifying recombinant proteins. There are several types of chromatography, each with their own advantages and disadvantages, including: Affinity chromatography, which uses a specific binding interaction between the protein of interest and a ligand or antibody that has been attached to a solid support. Ion exchange chromatography, which uses electrostatic interactions between the protein and a charged solid support to separate proteins based on their charge. Size exclusion chromatography, which separates proteins based on their size by passing them through a column filled with beads of a specific size. Hydrophobic interaction chromatography (HIC), which uses hydrophobic interactions between the protein and a hydrophobic solid support to separate proteins based on their hydrophobicity. Depending on the specifics of the protein and the downstream application, multiple chromatography steps may be necessary. It is important to be able to optimize the purification process to obtain the desired purity and yield of the protein. After chromatography, the protein can be further purified by methods such as ultrafiltration or diafiltration to remove any remaining contaminants. The purified protein can then be used for downstream applications such as enzymatic assays, structural studies, or as therapeutics.
The most common approach for protein purification is affinity chromatography, which separates proteins based on their unique interaction with a matrix. It is one of the most effective approaches because it uses the insertion of the desired structure (called a tag, such as His-tag or GST-tag) onto the protein. This tag is not found in any other molecule in the sample, giving our target protein special features that will be used to recognize and separate it from the others. However, in some cases, we cannot add a tag to our molecule and must resort to less specific ways, although they can be equally effective if used correctly.
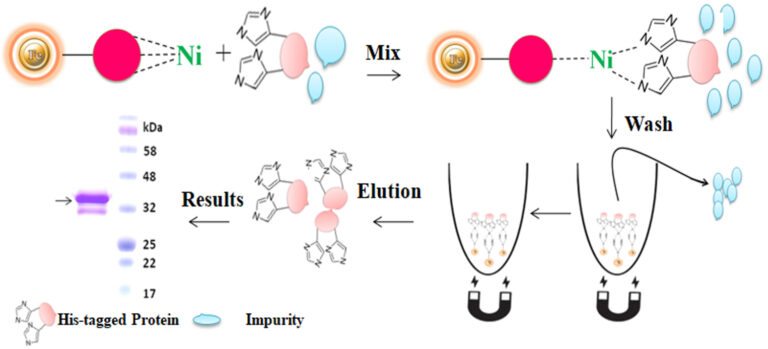
A polyhistidine tag called 6xHis-tag, His-tagged, and His-tag is a versatile tool that purifies highly pure recombinant protein from various expression systems, including bacterial, yeast, plant cell, and mammalian cell systems. The tag consists of six or more histidine residues positioned at either N or C terminus of a recombinant protein. Due to its small size, His-tag has several distinctive features, including less immunogenicity, hydrophilic nature, and versatility under native and denaturing conditions. Additionally, it is unnecessary to cleave the tag from the recombinant protein since it rarely perturbs the structure and function of its fusion protein. The purification principle of the His-tag is based on immobilized metal ion affinity chromatography (IMAC).
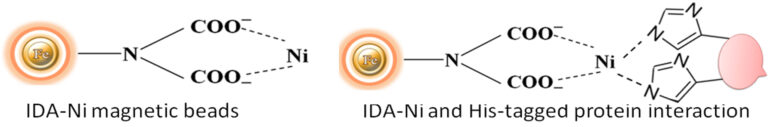
Although IMAC is a very effective protein purification technique, they are mainly made from traditional affinity chromatography matrices such as agarose resin or column. The problems with their procedures are tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. We developed an extremely efficient magnetic IMAC separation system to overcome these limitations.
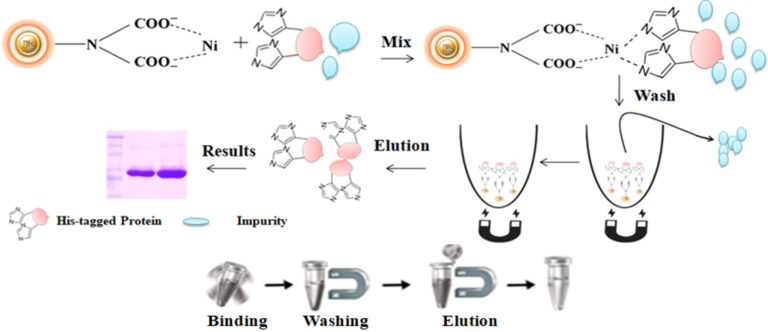
Magnetic beads (particles) are an entirely different type of solid support matrices from beaded agarose or other porous resins. They are much smaller (typically 1-5 µm diameter), thus providing larger surface areas for a high density of ligand immobilization. The beads are manufactured using nanometer-scale superparamagnetic iron oxide as the core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide. Pure inert silica makes less nonspecific binding. The purification with magnetic beads is straightforward. Mix the magnetic beads with the sample and incubate with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure of his tagged recombinant proteins are eluted by imidazole.
Advantages of magnetic particles over other matrices
●
Magnetic beads exhibit less nonspecific binding than porous supports.
●
Stable covalent bond with minimal ligand leakage
●
Direct purification from cell lysate without performing sample treatment.
●
Cost-effective: Eliminates columns, filters, laborious repeat pipetting, and organic reagents.
●
High-throughput: Compatible with many different automated liquid handling systems.
Workflow
1.
Harvest the cell by centrifuge
2.
3.
Mix the beads with the cell lysates.
4.
Wash the beads to remove unbound molecules.
5.
Elute the His-tagged protein with imidazole or EDTA-containing buffer or the acid buffer.
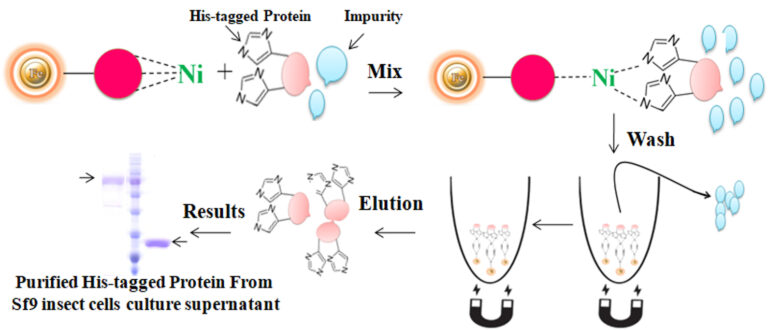
Culture medium compatible His-tagged protein purification
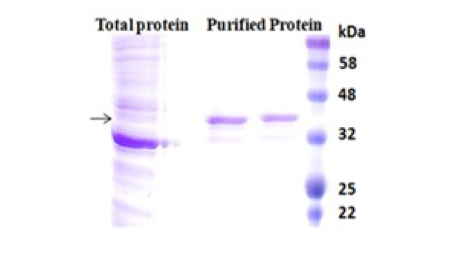
BcMag™ Secreted His-tagged protein purification system is based on magnetic beads coupled with a unique, proprietary ligand that is loaded with nickel ions. The ligands are extraordinarily firmly bonded and high affinity for His-tagged proteins. They exhibit low ion-leaching properties even in chemical additives such as chelators (EDTA), strong reducing agents (DTT), or components of cell culture supernatants, which typically strip off Ni ions and reduce the functionality of most IMAC magnetic beads. His-tagged protein purification resins allow the efficient purification of recombinant polyhistidine-tagged proteins directly from a soluble intracellular protein extract, HeLa, CHO mammalian cells, or Sf9 insect cells culture supernatant. They can be used manually with a magnetic stand or automatically with an instrument. It avoids extensive and time-consuming sample pretreatment processes, such as buffer exchange by dialysis in conjunction with concentration operations.
Nonspecific binding was a potential problem with the purification of his-tagged proteins from various expression systems, especially from the lower expressed recombinant his-tagged proteins or higher expressed proteins interacting with other cellular proteins. Many factors can cause nonspecific binding. Among them, low-expressed protein or the tagged protein bound with endogenous proteins could result in severely nonspecific binding. The binding of His-tagged proteins to the metal ion of the IMAC depends on electric charges. The nonspecific binding occurs when the his-tagged binding sites of resin are only partially bound by the protein of interest due to its low abundance. The rest of the binding sites non-specifically interacted with other slightly charged proteins, such as histidine-rich protein, leading to impurities later on. Multiple lines of evidence prove that reducing reagents and nonionic detergents in the binding and washing buffers could dramatically reduce the highly nonspecific binding and get purer proteins. The most used chelators in IMAC applications are iminodiacetate (IDA) or nitrilotriacetic acid (NTA). These chelators cannot use together with reducing reagents such as DTT (dithiothreitol), β-me ( β-Mercaptoethanol), and TCEP (TRIS (2-carboxyethyl) phosphine) since the reducing reagents can strip off the metal from these resins, resulting in the rapid loss of the protein binding capacity.
Moreover, those chelators are mainly immobilized to the traditional affinity chromatography matrices such as agarose resin or column. These solid matrices make the purification process tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. Bioclone introduces a powerful magnetic beads-based IMAC system to address these problems.
BcMag™ Low Expression His-Tagged Protein Purification Kit uses unique reducing reagent-compatible magnetic beads to purify low-expressed protein, or the target protein bound with the cellular protein.
Moreover, those chelators are mainly immobilized to the traditional affinity chromatography matrices such as agarose resin or column. These solid matrices make the purification process tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. Bioclone introduces a powerful magnetic beads-based IMAC system to address these problems.
Workflow
The purification with magnetic microparticles is straightforward. Mix the microparticles with the sample and incubate them with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure His-tagged recombinant proteins are eluted by imidazole.
Feature and benefits
●
Magnetic beads exhibit less nonspecific binding than porous supports.
●
Stable covalent bond with minimal ligand leakage
●
The beads resist up to 20 mM EDTA and 20 mM reducing reagents without nickel leaching.
●
High protein purity
●
Cost-effective: Eliminates columns, filters, repeat pipetting, and organic reagents.
●
High-throughput: Compatible with many different automated liquid handling systems.
Applications
●
Investigating protein structure and function
●
Preparing recombinant proteins for X-ray crystallography
●
Ideal for the study of protein interactions with protein or DNA
●
Immunization to raise antibodies against a protein of interest
●
Effective screening protein expression, even with crude cell lysates
●
Microscale purification of his-tagged proteins
Explore
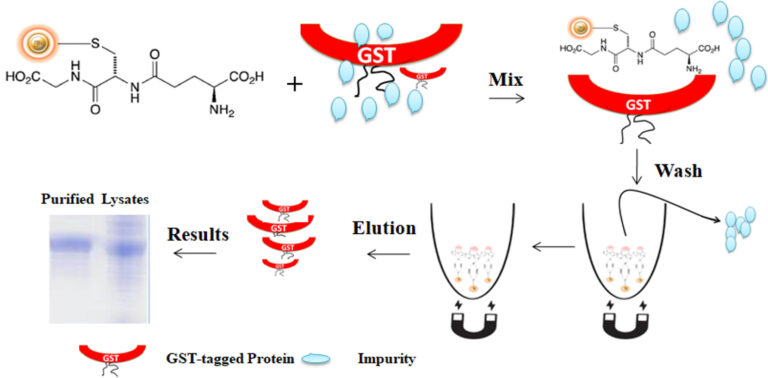
Glutathione-S-transferase (GST) is a highly soluble and stable 26 kDa enzyme that catalyzes the protective mechanisms of Glutathione (GSH). Many eukaryotic proteins are produced as inclusion bodies (insoluble aggregated protein), a malfunctioning protein caused by misfolding, in prokaryotic expression systems such as E.coli. It is usually painful to refold the inclusion body into a functional protein. GST is widely used as a fusion partner that promotes greater expression and solubility of the desired protein by taking advantage of its high stability and solubility to overcome this problem. Moreover, GST has a high affinity toward reduced Glutathione, its natural substrate. Therefore, GST as an affinity tag becomes a versatile tool for single-step purification of active recombinant production in a prokaryotic expression system.
Glutathione is a short peptide (Glu-Cys-Gly) with a high affinity toward glutathione S-transferase (GST). The matrix can precisely capture GST-tagged protein via the affinity interaction when the Glutathione is immobilized in a chromatography matrix such as beaded agarose or magnetic beads. The GST tag can fuse to either the C- or N-terminus of a protein by inserting DNA sequence coding for the protein of interest into commercial expression vectors. If desired, the protein of interest can be cleaved off the GST tag by site-specific protease. The protease site can be engineered between the GST tag and the protein of interest.
Glutathione is mainly immobilized to the traditional affinity chromatography matrices such as agarose resin or column. These solid matrices make the purification process tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. Bioclone introduces a powerful magnetic beads-based GST–tagged protein system to overcome these problems.
BcMag™ GST-Tagged Protein Purification Magnetic Beads are magnetic microspheres covalently immobilized with a high density of Glutathione. The microspheres combine all the advantages of affinity protein purification (low costs, simplicity, high specificity, and capacity) and magnetic properties to perform efficient manual or automatic quick high-throughput purification. It is specially designed for the capture and purification of GST-tagged proteins from various sample types.
Workflow
The purification with magnetic microparticles is straightforward. Mix the microparticles with the cell lysates and incubate them with continuous rotation for a sufficient time. The beads remain suspended in the sample solution during mixing, allowing the GST-tagged protein to bind to the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure GST-tagged recombinant proteins are eluted by excess reduced Glutathione.
Feature and benefits
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Stable covalent bond with minimal ligand leakage
●
High binding capacity, very low nonspecific binding
●
Scalable – Easily adjusts for sample size and automation
●
Reproducible results
Applications
●
Investigating protein structure and function
●
Preparing recombinant proteins for X-ray crystallography
●
Ideal for the study of protein interactions with protein or DNA
●
Immunization to raise antibodies against a protein of interest
●
Effective screening protein expression, even with crude cell lysates
●
Microscale purification of GST-tagged proteins
Explore
Gel filtration is one of these methods, which separates molecules based on their size. Molecules can be sorted by their difficulty when passing through a resin with holes of a given diameter. Ion exchange chromatography is another widely used technology that separates molecules based on their electric charge under specific pH and temperature conditions. Hydrophobic interaction chromatography and reverse-phase chromatography are the most often used methods for separating proteins depending on their polarity. The critical distinction between these approaches is the polarity of the matrix with which the purified protein interacts.
Explore
Recombinant proteins are extensively utilized in the production of pharmaceuticals, protein-based polymers for drug administration, antibodies and enzymes for disease therapy, protein scaffolds for tissue engineering, and various other applications.
Recombinant proteins now account for most top-selling medications, including those used to treat complicated disorders ranging from arthritis to cancer and battle infectious diseases such as COVID-19 by neutralizing antibodies.
Recombinant pathogenic protein-based serological assays may attain excellent sensitivity and specificity because of the high concentration of immunoreactive antigens and the lack of nonspecific moieties present in whole-cell preparations from the blood of experimentally infected animals. A vaccinated person creates antibodies against the protein antigen, which protects them from acquiring the disease when the pathogenic microorganism attacks.
Recombinant protein vaccines have a long history in the industry, dating back to the mid-1980s with the hepatitis B vaccine, which is now a common vaccination worldwide. These vaccines represented the first step away from traditional manufacturing, overcoming numerous difficulties in vaccine development and manufacture. Unlike antigen purification from an inactivated pathogen, recombinant antigen synthesis allows for high expression levels and increases vaccination safety.
Explore
1.
Imataka H, Mikami S (2009) Advantages of human cell-derived cell-free protein synthesis systems. Seikagaku 81(4):303–7.
2.
Mikami S et al. (2008) A human cell-derived in vitro coupled transcription/translation system optimized for production of recombinant proteins. Protein Expr Purif 62(2):190–8.
3.
Kobayashi T et al. (2007) An improved cell-free system for picornavirus synthesis. J Virol Methods 142(1-2):182–8.
4.
5.
6.
Oliveira C, et al. (2018) Guidelines to reach high-quality purified recombinant proteins. Appl Microbiol Biotechnol 102(1): 81-92.
7.
Kosobokova EN, et al. (2016) Overview of fusion tags for recombinant proteins. Biochemistry (Mosc) 81(3): 187-200.
8.
Wingfield PT (2015) Overview of the purification of recombinant proteins. Curr Protoc Protein Sci 80: 6.1.1-6.1.35.
9.
Assenberg R, et al. (2013) Advances in recombinant protein expression for use in pharmaceutical research. Curr Opin Struct Biol 23(3): 393-402.
10.
Ohba Y, et al. (2013) Fluorescent protein-based biosensors and their clinical applications. Prog Mol Biol Transl Sci 113: 313-348.
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.