- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Magnetic bead separation is a fast, effective, and clean method used by scientists to replace filtering, centrifugation, and separation processes. Time-resolved Fluorescence Magnetic Beads can be used for immunoassays and other applications. They have high surface-to-volume ratios, small sizes (0.1-10µm), various functional groups attached to the surfaces (e.g., antibodies, DNA, and chemical groups), and the ability to manipulate the particles via an applied magnetic field easily. Combined with automated liquid handling and robust detection instrumentation, these characteristics enable a wide range of high-throughput applications.
BcMag™ Magnetic Beads are monodispersed and core−shell superparamagnetic beads. The beads are uniform in size, have a narrow size distribution with a large surface area, and have a unique surface coating with excellent batch-to-batch performance. They are available in 1µm, 2.5 µm, and 5.0 µm sizes. The beads are manufactured using nanometer-scale superparamagnetic iron oxide as the core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide. The excellent hydrophilic properties of the beads make them less nonspecific binding. Unlike most commercially available magnetic beads based on hydrophobic materials such as polystyrene, silica provides an ideal chromatographic separation medium for the purification of target molecules due to its mechanical and chemical stability.
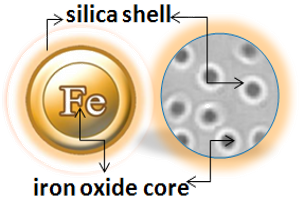
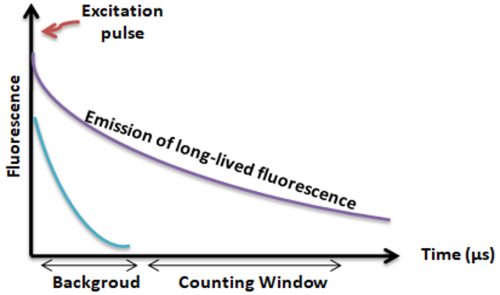
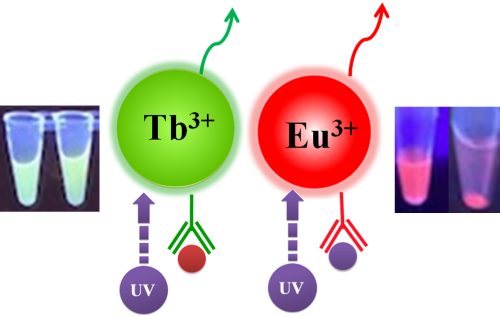
Table 1. Fluorescence Properties of TRF Magnetic Beads
Fluorophore
Red
Green
Far-Red
340
320
470
615
545
710
Fluorescence lifetime (Ԏ) (µsec)
730
1050
354.36
Stokes shifts
(nm)
275
220
175
Fluorophore
Fluorescence lifetime (Ԏ) (µsec)
Stokes shifts
(nm)
Red
340
615
730
275
Green
320
545
1050
220
Far-Red
470
710
354.36
175
Applications
TR-FRET (Time-Resolved FRET) is a well-known technology widely used in clinical diagnostics and academic laboratories. TR-FRET has proven to be a highly versatile assay approach, allowing researchers to study a wide range of biological interactions with low to high affinity, using both small and large molecules. BcMag™ Time-resolved Fluorescence Magnetic Beads as donors can be used in a TR-FRET assay.
Learn More
1.
Perform a double function simultaneously on the same beads: The magnetic beads combine separation/preconcentration and detect analytes, allowing quick, simple, robust, and high-throughput analytes of trace amounts from complex biological samples on the same beads.
2.
Ultra sensitive. Lower detection limits of 10 pg/mL versus typical fluorometric detection limits of 100 pg/mL.
3.
Extremely photostable and highly resistant to photobleaching. All the lanthanides chelate or cryptate molecules and iron oxide are entirely encapsulated inside each bead instead of merely on the bead’s surface. The protective environment prevents iron oxide and dye from leaching into aqueous media, which makes the beads less sensitive to external conditions such as solvent, temperature, pH, etc.
4.
Very high Fluorescence intensity. Because a single bead has a large concentration of lanthanide chelate with a high quantum yield ranging from 40 to 90%, the beads show excellent fluorescence intensity, which increases test sensitivity without signal amplification. Such bright beads are also perfect for donors’ use in time-resolved FRET assays.
5.
Lanthanides chelate or cryptate has large Stokes shifts (>250 nm), narrow emission bands (-10 nm bandwidth), and long fluorescence lifetime (μs), which dramatically reduces background and increases the signal-to-noise ratio.
6.
Most bioprocess ELISA assays can be converted to an HTRF assay.
7.
No washing step is involved in the assays.
8.
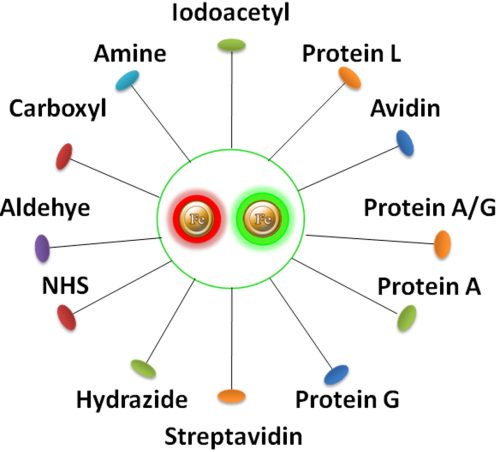
Have a hydrophilic silica surface grafted by different functional groups with linkers of variable lengths, allowing efficient conjugation of various ligands such as peptides, proteins, antibodies, small molecules, carbohydrates, aptamers, DNA/RNA, etc.
9.
Due to the microsphere’s magnetic properties, the Fluorescence magnetic beads are suitable for high-throughput automation.
BcMag™ NHS-Activated TR Magnetic Beads are uniform, magnetic beads coated with high-density NHS (N-hydroxyl succinimide) functional groups on the surface. The magnetic resin utilizes reliable NHS-ester chemistry and does not require the use of dangerous chemicals for immobilization. The beads have less nonspecific binding and no leaking of the coupled ligand. The resin can quickly, efficiently, and covalently conjugate any primary amine-containing ligands by forming stable amide linkages with better than 85% coupling efficiency. Bioclone NHS-Activated beads reactions take less than an hour (15–30 min at room temp pH 7–9, 4 hours at 4 °C) and produce substantially more stable linkages.
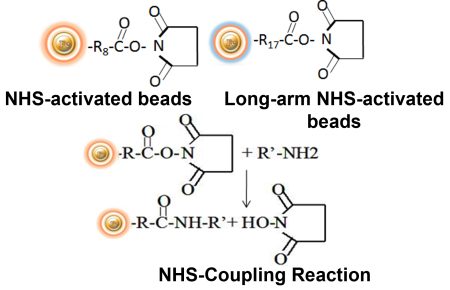
Explore Products
Labeling Protocol
BcMag™ Aldehyde-Activated TR Magnetic Beads are uniform, silica-based superparamagnetic beads grafted with a high density of aldehyde functional groups on the surface. The bead aldehyde groups react spontaneously with primary amines present at the N-terminus of proteins or in lysine residues to form intermediate Schiff Base complexes. The reaction of reductive amination immobilization starts with the creation of an initial Schiff base between the aldehyde and amine groups, which is then reduced to a secondary amine by the addition of sodium cyanoborohydride (NaCNBH3) to generate stable amine bonds between the bead and the ligand. At physiological to alkaline circumstances (pH 7.2 to 9) in either aqueous or organic solvents with 20% ~ 30% DMSO or DMF, coupling reaction takes 2 to 6 hours in a one-step process. Coupling efficiency with antibodies and normal proteins is usually better than 85%, resulting in 15 – 20 µg /mg of beads.
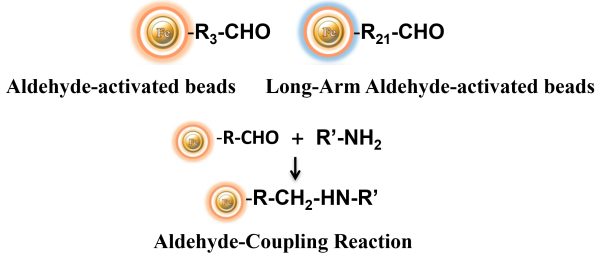
The TRF Magnetic Beads are suitable for conjugating a large protein. The Aldehyde-Activated resins have higher coupling efficiency than cyanogen bromide (CNBr) activated supports. Furthermore, the aldehyde chemistry generates an uncharged connection with the amine-containing ligand that is more stable than the CNBr approach. The hydrophilic surface ensures excellent dispersion and easy handling in various buffers. When utilized for affinity purification methods, these features allow better leak-resistant immobilization and lower nonspecific binding.
Explore Products
Labeling Protocol
It is frequently helpful to immobilize affinity ligands via functional groups other than amines. The thiol group, in particular, can be employed to guide coupling processes away from active centers or binding sites on specific protein molecules. Sulfhydryls, commonly known as thiols, are found in proteins as side chains of cysteine (Cys, C) amino acids. As the foundation of native tertiary or quaternary protein structure, pairs of cysteine sulfhydryl groups are frequently linked via disulfide bonds (-S-S-) within or between polypeptide chains. For most thiol-reactive compounds, only free or reduced sulfhydryl groups (-SH) [rather than sulfur atoms in disulfide bonds] are available for reaction.
Advantages of protein conjugation via sulfhydryl groups:
First, sulfhydryls are found in most proteins but are not as abundant as primary amines; immobilizing via sulfhydryl groups is more selective and precise. Second, because sulfhydryl groups in proteins are frequently engaged in disulfide bonds, crosslinking at these locations usually affects the underlying protein structure or blocks binding sites. Third, the quantity of accessible (i.e., free) sulfhydryl groups can be easily regulated or manipulated; they can be formed by reducing native disulfide bonds or introduced into molecules via reactivity with primary amines. Finally, combining sulfhydryl-reactive groups with amine-reactive groups to create heterobifunctional crosslinkers allows for more flexibility and control over crosslinking methods.
BcMag™ Iodoacetyl-activated TR Magnetic Beads are uniform, silica-based superparamagnetic beads coated with a high density of Iodoacetyl functional groups on the surface. It is designed to enable fast, efficient, and covalent immobilization of proteins, peptides, and other ligands through their sulfhydryl groups (-SH) for affinity purification procedures. At physiological to alkaline circumstances (pH 7.2 to 9) in either aqueous or organic solvents 20- 30% DMSO or DMF, iodoacetyl-activated supports react with sulfhydryl groups, resulting in stable thioether bonds. These reactions are often carried out in the dark to prevent the formation of free iodine, which can react with tyrosine, histidine, and tryptophan residues (Fig.7). The hydrophilic surface ensures the bead’s low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
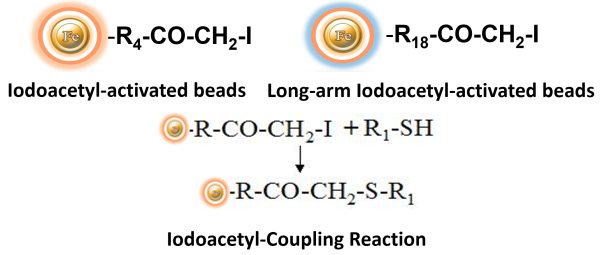
Explore Products
Labeling Protocol
In their natural condition, most biological compounds do not contain carbonyl ketones or aldehydes. However, similar groups of proteins may be effective for forming an immobilization site that drives covalent coupling away from active centers or binding regions. Glycoconjugates, such as glycoproteins and glycolipids, typically contain sugar residues with hydroxyls on neighboring carbon atoms; these cis-diols can be oxidized with sodium periodate to produce aldehydes as covalent immobilization sites.
BcMag™ Hydrazide Magnetic Beads are uniform magnetic beads grafted with a high density of hydrazide functional groups on the surface. Hydrazide chemistry is effective for labeling, immobilizing, or conjugating glycoproteins via glycosylation sites, which are frequently (as with most polyclonal antibodies) positioned in domains distant from the critical binding sites whose function needs to be preserved. Coupling antibodies in this manner selectively targets heavy chains in the Fc portion of the molecule, assisting in the best possible preservation of antigen binding activity by the ends of the Fv regions. At pH 5 to 7, hydrazide-terminated supports and compounds will conjugate with carbonyls of oxidized carbohydrates (sugars), producing hydrazone linkages.
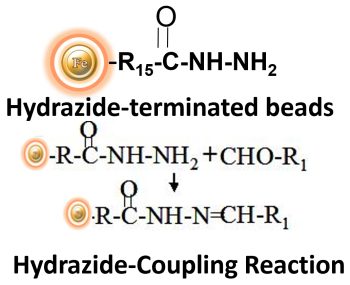
Explore Products
Conjugation Protocol
The carboxyl group is found in a wide range of biological substances. Peptides and proteins contain carboxyls (-COOH) at the C-terminus of each polypeptide chain, as well as aspartic acid (Asp, D) and glutamic acid side chains (Glu, E). Carboxyls, like primary amines, are typically found on the surface of protein structures.
Carboxylic acids can be employed to immobilize biological molecules by applying a carbodiimide-mediated process. Although no activated support has a reactive group that is spontaneously reactive with carboxylates, chromatographic supports containing amines (or hydrazides) can be utilized to create amide bonds with carboxylates activated using the water-soluble carbodiimide crosslinker EDC.
BcMag™ Amine-activate TR Magnetic Beads are magnetic beads coated with a high density of primary amine functional groups on the surface. The beads can covalently conjugate primary amine or carboxy-containing ligands such as protein and peptides for affinity purification. The hydrophilic surface ensures beads have low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
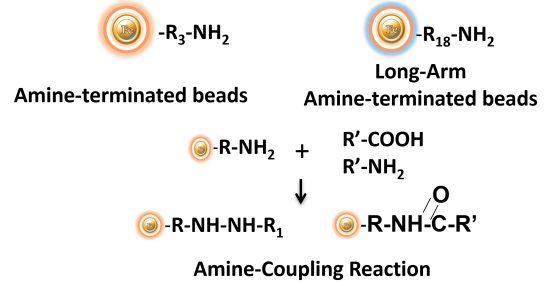
Explore Products
Labeling Protocol
BcMag™ Carboxyl-activated TR Magnetic Beads are uniform, silica-based magnetic beads coated with a high density of carboxylic acid groups on the surface. The hydrophilic surface ensures bead’s low nonspecific adsorption, excellent dispersion, and easy handling in various buffers. The high density of pendent functional carboxylic acid groups on their surface allows covalently conjugate primary amine-containing ligands via a stable amide bond. Moreover, the hydrophilic surface ensures beads low nonspecific adsorption, excellent dispersion, and easy handling in various buffers.
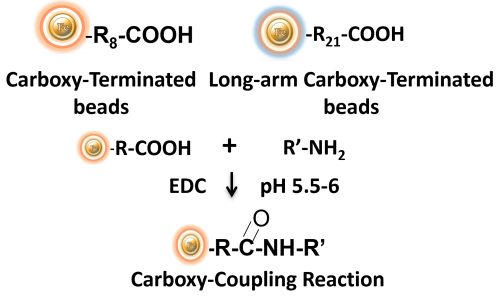
EDC and other carbodiimides are zero-length crosslinkers that cause carboxylates (-COOH) to conjugate directly to primary amines (-NH2) without becoming part of the ultimate crosslink (amide bond) between target molecules. Ligand immobilization occurs when the amine-terminated resin is employed as the primary amine in this process.
Because peptides and proteins contain numerous carboxyls and amines, direct EDC-mediated crosslinking typically results in random polypeptide polymerization. However, this reaction chemistry is commonly utilized in immobilization methods (for example, binding proteins to a carboxylated surface) and immunogen production (e.g., attaching a small peptide to a large carrier protein). Indeed, some peptide polymerization is advantageous in antibody manufacturing and purification operations because it boosts overall peptide conjugation (loading) and ensures that the peptide ligands are supplied in numerous orientations.
Explore Products
Labeling Protocol
BcMag™ Protein A/G TRF Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres that are covalently coupled with Protein A/G on the surface. The microspheres combine the benefits of a novel antibody-binding protein, time-resolved Fluorescence dyes, and magnetic characteristics to perform sensitive assays. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and europium metal as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and europium metal.
Protein A/G is a genetically engineered recombinant fusion protein that includes four Fc binding domains from Protein A and two from Protein G and has a final mass of 50.4 kDa. Proteins A and G are antibody-binding proteins derived from Staphylococcus aureus and Streptococcus sp., respectively. These proteins have different immunoglobulin-binding capacities and specificities. Protein A/G is a more potent protein exhibiting less pH-dependent binding and has binding profiles of both proteins compared to Protein A or Protein G alone. Protein A/G strongly binds to human total IgG, IgG1, IgG2, IgG3, and IgG4, which is ideal for binding polyclonal or monoclonal IgG antibodies, whose subclasses are unsure yet. In the case of mouse IgG, Protein A/G can bind to all classes, including total IgG, IgG1, IgG2a, IgG2b, and IgG3 – except IgA, IgM, and serum albumin, allowing Protein A/G to efficiently purify and detect mouse monoclonal IgG antibodies, without cross-binding to IgA, IgM and serum albumin. Also, Protein A/G binds more powerfully and efficiently to mouse monoclonal antibodies than to Protein A or Protein G alone.
Explore Products
BcMag™ Protein A TRF Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres that are covalently coupled with Protein A on the surface. The microspheres combine the benefits of a novel antibody-binding protein, time-resolved Fluorescence dyes, and magnetic characteristics to perform very sensitive assays. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and TRF dyes as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and TRF dyes.
Protein A is an antibody-binding cell wall protein that originates from Staphylococcus aureus. It comprises a single polypeptide chain with little or no carbohydrates and has a molecular mass of 42kDa. It consists of a signal sequence, five immunoglobulin-binding domains E-D-A-B-C aligned in series, each of which binds strongly to the heavy chain constant region (Fc) of IgG H2-CH3 from various mammalian species, and a cell-wall binding domain. The recombinant protein has a higher capacity and maximum specific IgG binding than native Protein A since it only keeps the IgG-binding domains. Protein A binds to antibodies from various animals, including mice, humans, rabbits, pigs, dogs, and cats.
Explore Products
BcMag™ Protein G TRF Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres that are covalently coupled with Protein A on the surface. The microspheres combine the benefits of a novel antibody-binding protein, time-resolved Fluorescence dyes, and magnetic characteristics to perform very sensitive assays. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and TRF dyes as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and TRF dyes.
The beads contain the recombinant Protein G with 200 amino acids having a molecular mass of 21.8kDa with two Fc-binding domains per protein. These domains can bind to antibodies from many species, including mice, humans, rabbits, cows, goats, and sheep. The albumin and cell surface binding domains have been removed from Recombinant Protein G to reduce nonspecific binding. This recombinant protein has a significantly greater capacity and more specific IgG binding than native Protein G and Protein A for most mammalian IgGs. It can purify mammalian IgGs that do not bind well to Protein A. In addition, when working with complicated biological samples, the beads blocked by our unique blocking agent minimize and reduce nonspecific binding to the bead surface.
Explore Products
BcMag™ Protein L TRF Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres that are covalently coupled with Protein A/G on the surface. The microspheres combine the benefits of a novel antibody-binding protein, time-resolved Fluorescence dyes, and magnetic characteristics to perform very sensitive assays. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and TRF dyes as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and europium metal.
Protein L is a 36 kDa immunoglobulin-binding protein isolated from the anaerobic species Peptostreptococcus magnus but is now produced recombinantly in large amounts by E.coli. The recombinant Protein L contains only the IgG-binding domain; the other binding domains (Albumin, cell wall, and cell membrane) have been removed to ensure the maximum and specific binding properties. Protein L binds the different antibody isotypes (IgG, IgM, IgA, IgE, and IgD) through the interaction with the variable domain of the Ig kappa light chain with no interference with an antibody’s antigen-binding site. Compared with Protein A, Protein G, or Protein A/G, Protein L binds a broader range of antibody classes than Protein A or G since the heavy chain is not involved in the binding interaction. Protein L binds to most classes of Ig and single-chain antibody fragments (scFv) and Fab fragments.
Explore Products
BcMag™ Avidin TRF Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres that are covalently coupled with avidin protein. The microspheres combine the benefits of a novel avidin biotin-binding method, time-resolved Fluorescence dyes, and magnetic characteristics to perform very sensitive assays. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and europium metal as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and europium metal.
Avidin
Avidin is a biotin-binding glycosylated protein derived from chicken white. It comprises four 128 amino acid subunits, each binding one biotin molecule over a wide range of pH and temperature. Since avidin can be chemically modified with little to no effect on its function and is easily isolated from chicken egg whites, it helps detect biotinylated molecules in various conditions.
Explore Products
BcMag™ Streptavidin-TRF Magnetic Beads are Time-Resolved Fluorescence (TRF) magnetic microspheres that are covalently coupled with streptavidin protein. The beads are manufactured using nanometer-scale superparamagnetic iron oxide and europium metal as core and entirely encapsulated by a high-purity silica shell, ensuring no leaching problems with the iron oxide and TRF dyes. The microspheres combine the benefits of a novel streptavidin biotin-binding method, time-resolved Fluorescence dyes, and magnetic characteristics to perform very sensitive assays.
Streptavidin is a tetrameric biotin-binding protein that originates from Streptomyces avidin and has a mass of 60 kDa. It has no carbohydrates and lower pI, giving a lower degree of nonspecific binding, making streptavidin an ideal reagent choice for many detection systems.
Streptavidin and its analogs have become powerful tools for probes and affinity ligands for various applications in biochemical assays, diagnosis, affinity purification, and drug delivery.
Explore Products
1.
Corr SA, Rakovich YP, Gun’ko YK. Multifunctional Magnetic-Fluorescence Nanocomposites for Biomedical Applications. Nanoscale Res Lett. 2008 Mar 6;3(3):87–104.
2.
Diamandis EP 1988. Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem. 1988 Jun;21(3):139-50.
3.
Fan W, Liu D, Ren W, Liu C. Trends of Bead Counting-Based Technologies Toward the Detection of Disease-Related Biomarkers. Front Chem. 2020 Dec 21;8:600317.
4.
Leng, Y., Sun, K., Chen, X., and Li, W. (2015). Suspension arrays based on nanoparticle-encoded microspheres for high-throughput multiplexed detection. Chem. Soc. Rev. 44, 5552–5595
5.
Ma, F., Li, Y., Tang, B., and Zhang, C. (2016). Fluorescence biosensors based on single-molecule counting. Acc. Chem. Res. 49, 1722–1730
6.
Parsa, S. F., Vafajoo, A., Rostami, A., Salarian, R., Rabiee, M., Rabiee, N., et al. (2018). Early diagnosis of disease using microbead array technology: a review. Anal. Chim. Acta 1032, 1–17.
7.
Werts, M. H. V. (2005). “Making Sense of Lanthanide Luminescence.” Science Progress. 88 (2): 101–131
8.
Rödiger, S., Liebsch, C., Schmidt, C. et al. Nucleic acid detection based on the use of microbeads: a review. Microchim Acta 181, 1151–1168 (2014).
9.
Soini, Erkki; Lövgren, Timo; Reimer, Charles B. (January 1987). “Time-Resolved Fluorescence of Lanthanide Probes and Applications in Biotechnology”. C R C Critical Reviews in Analytical Chemistry. 18 (2): 105–15
10.
Satoshi Sakamoto, Kenshi Omagari, Yoshinori Kita, Yusuke Mochizuki, Yasuyuki Naito, Shintaro Kawata, Sachiko Matsuda, Osamu Itano, Hiromitsu Jinno, Hiroya Takeuchi, Yuki Yamaguchi, Yuko Kitagawa, Hiroshi Handa, Magnetically Promoted Rapid Immunoreactions Using Functionalized Fluorescence Magnetic Beads: A Proof of Principle, Clinical Chemistry, Volume 60, Issue 4, 1 April 2014, Pages 610–620
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.