- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Specification
Composition
Magnetic beads grafted with the sulphonic acid functional groups.
Number of Beads
~ 1.68 x 109 beads/mg (1μm beads)
~ 5 x 107 beads /mg (5μm beads)
Magnetization
~45 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.0 g/ml
Stability
Most organic solvents
Strong Cation Exchange Beads
1.0 μm beads: ~2.5 mg Lysozyme / ml of Beads
5 μm beads: ~2 mg Lysozyme / ml of Beads
Storage
Store at 4°C upon receipt.
The utilization of Strong Cation Exchange magnetic resins has revolutionized the realm of scientific research by providing a feasible alternative to the laborious, complicated, and exorbitant chromatographic procedures like agarose, cellulose, sepharose, and Sephadex-based columns or resins. The traditional column-based approaches require centrifugation or clearance of the lysate, addition of supernatant to the column, washing of the membrane or resin with buffer through centrifugation or vacuum manifold, and the elution of requisite biomolecules in an appropriate volume of buffer. However, the time-consuming and tedious pipetting involved in processing multiple samples in academic research labs may lead to inconsistencies in the target biomolecule yield between experiments and individuals. This makes it necessary for staff and students to undergo extensive training and practice to ensure a constant protein yield.
Strong Cation Exchange Magnetic resins have emerged as a new technology, providing numerous advantages over non-magnetic resin approaches. The magnetic beads’ ease of use, rapid experimental protocols, suitability, and convenience for high-throughput automated and miniaturized processing has seen an exponential increase in their application across different areas of life-sciences research and development. These include drug discovery, biomedicine, bioassay development, diagnostics, genomics, and proteomics.
The BcMag™ Strong Cation Exchange (SCX) Magnetic Beads are a uniform magnetic bead grafted with strong cation exchangers (sulphonic acid functional groups) on the surface, providing a perfect solution to the drawbacks of traditional chromatographic methods. The magnetic resin-based format allows for quick high-yield processing of 96 samples in roughly 20 minutes, thus minimizing the tedious pipetting required in the traditional approaches. It enables rapid and efficient fractionation of proteins or nucleic acids from complex biological samples (such as serum, plasma, etc.) manually or automatically. The purified protein can be utilized in downstream applications such as sample fractionation for 1D and 2D SDS-PAGE, X-ray crystallization, and NMR spectroscopy. Besides, Strong ion exchangers are an effective separation tool when weak ion exchangers fail, given that the selectivities of weak and strong ion exchangers frequently differ.
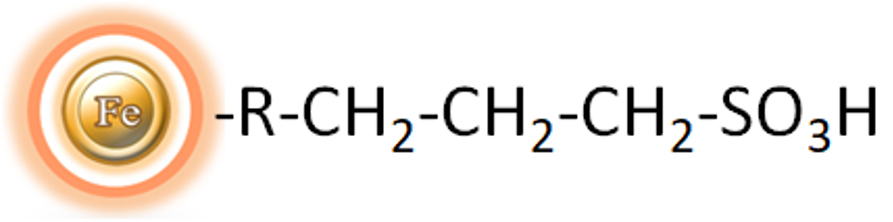
●
Fast and straightforward – Magnetic beads-based format eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Convenient and expandable – Magnetic format enables high-throughput processing of multiple samples in parallel with many different automated liquid handling systems.
●
Robust – Magnetic beads do not crack or run dry.
●
Low bed volume – Working with small magnetic bead volumes allows for minimal buffer volumes, resulting in concentrated elution fractions.
●
Protein pre-fractionation in cell lysates
●
Optimizing purification conditions for new protein preparation protocols
●
Protein purification and concentration
●
Antibody purification from serum, ascites, or tissue culture supernatant
●
Preparation of samples before 1D or 2D PAGE
●
Phosphopeptide purification before MS analysis
Note: The following protocol is an example of fractionating a protein or peptide sample with BcMag™ SCX magnetic beads. Users are encouraged to choose alternative binding, washing, or elution buffers to get the best results and determine the optimal working conditions based on the protocol and suggestions described in the troubleshooting section. It is critical to match the amount of the resins to the amount of protein in the starting material in all protein purification experiments. It is not only for financial reasons but also because insufficient SCX resin results in inadequate protein binding in the solution. Too many affinity binding sites will result in the binding of other proteins, making the purification less selective and requiring extra purification steps to achieve pure protein. We recommend performing a titration to optimize the beads used for each application. It is necessary to optimize volumes of elution to avoid unnecessary sample dilution.
Note: Select the appropriate buffer
●
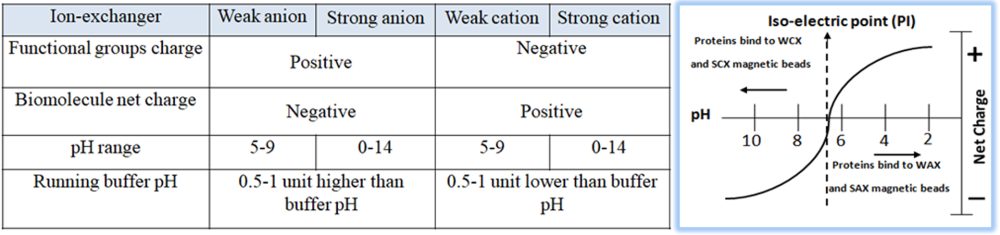
Based on the protein’s pI, empirically calculate the appropriate buffer (pH and salt concentration) for purifying and eluting the protein of interest. In a buffered solution above the protein’s pI, the protein becomes negatively charged (deprotonated) and binds to the positively charged functional groups of an anion exchange resin. To choose the correct buffer for a selected pH, the following is a general rule for selecting a buffer pH:
Anion exchanger — 0.5–1.5 pH units higher than the protein’s pI of interest.
Cation exchanger — 0.5–1.5 pH units lower than the protein’s pI of interest.
A. Equipment
●
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
For larger scale purification, Ceramic Magnets Block for large scale purification (6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8).
●
Corning 430825 cell culture flask for large-scale purification (Cole-Parmer, Cat. No. EW-01936-22)
●
Mini BlotBoy 3D Rocker, fixed speed, small 10″ x 7.5″ platform w/ flat mat (Benchmark Scientific, Inc. Cat. No. B3D1008) or compatible
B. Buffer
●
Binding/Wash Buffer: 25 mM Sodium acetate, pH 5.5
●
Elution Buffer: 50 mM Sodium phosphate, pH 8.0, 0.05-2.5 M NaCl
General Protocol for using the Strong Anion Exchange Magnetic Beads –
a.
Strong Cation Exchange magnetic beads preparation
1.
Vigorously shake the bottle until the magnetic beads become homogeneous and transfer an appropriate volume of the magnetic beads from the bottle to a new tube or flask.
Note:
●
Optimize the number of beads used for each application. Too many beads will cause higher background. Insufficient beads will lead to lower yields.
●
2.
3.
Repeat step (2) one more time.
4.
Equilibrate the beads by adding ten bead-bed volumes of Binding/Washing buffer and shake it to mix them. Incubate at room temperature with continuous rotation for 2 minutes. Place the tube on the magnetic separator for 1-3 minutes. Remove the supernatant while the tube remains on the separator. The beads are ready for purification.
b.
Purification
1.
Add the equilibrated beads (Step a (4)) to the sample and incubate on Mini BlotBoy 3D Rocker with continuous rotation for 5-10 minutes.
2.
Place the tube on the magnetic separator for 1-3 minutes. Remove the supernatant while the tube remains on the separator. Add ten bead-bed volumes of Binding/Washing buffer and shake it ten times to wash the beads. Again, place the tube on the magnetic separator for 1-3 minutes and remove the supernatant while the tube remains on the separator.
3.
Repeat step (2) six times.
Note:
●
This step is critical to get high pure protein. It may be necessary to wash the beads more than six times for some proteins to reduce the nonspecific binding.
●
Optimize the washing buffer (pH and salt concentration)
Elute protein with an appropriate volume of elution buffer by pipetting up and down 10-15 times or vortex mixer for 5 minutes.
Note:
Determine the optimum elution buffers (pH and salt concentration) and eluting the protein 2-3 times may be necessary.
4.
Elute protein with an appropriate volume of elution buffer by pipetting up and down 10-15 times or vortex mixer for 5 minutes.
Note:
Determine the optimum elution buffers (pH and salt concentration) and eluting the protein 2-3 times may be necessary.
5.
Collect and transfer the supernatant to a new tube.
C. Troubleshooting
Problem
Low yield
Probable Cause
The sample’s ionic strength is high.
Suggestion
Problem
Low yield
Probable Cause
The sample contains interfering detergents.
Suggestion
Problem
The protein failed to elute.
Probable Cause
Ionic interaction is too strong.
Suggestion
Problem
Poor separation
Probable Cause
Carry-over between eluted fractions
Suggestion
Problem
Poor separation
Probable Cause
Proteins or peptides with similar pI to the target protein
Suggestion
Problem
Probable Cause
Suggestions
Low yield
The sample’s ionic strength is high.
The sample contains interfering detergents.
The protein failed to elute.
Ionic interaction is too strong.
Poor separation
Carry-over between eluted fractions
Proteins or peptides with similar pI to the target protein
1.
Edelmann MJ. Strong cation exchange chromatography in analysis of posttranslational modifications: innovations and perspectives. J Biomed Biotechnol. 2011;2011:936508.
2.
Stone MT, Cotoni KA, Stoner JL. Cation exchange frontal chromatography for the removal of monoclonal antibody aggregates. J Chromatogr A. 2019 Aug 16;1599:152-160.
3.
Herciková J, Spálovská D, Frühauf P, Izák P, Lindner W, Kohout M. Design and synthesis of naphthalene-based chiral strong cation exchangers and their application for chiral separation of basic drugs. J Sep Sci. 2021 Sep;44(18):3348-3356.
4.
Janakiraman VN, Solé M, Maria S, Pezzini J, Cabanne C, Santarelli X. Comparative study of strong cation exchangers: Structure-related chromatographic performances. J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Mar 30;1080:1-10.
5.
Wang F, Dong J, Jiang X, Ye M, Zou H. Capillary trap column with strong cation-exchange monolith for automated shotgun proteome analysis. Anal Chem. 2007 Sep 1;79(17):6599-606.
6.
Das S, Bosley AD, Ye X, Chan KC, Chu I, Green JE, Issaq HJ, Veenstra TD, Andresson T. Comparison of strong cation exchange and SDS-PAGE fractionation for analysis of multiprotein complexes. J Proteome Res. 2010 Dec 3;9(12):6696-704.
7.
Steinebach F, Wälchli R, Pfister D, Morbidelli M. Adsorption Behavior of Charge Isoforms of Monoclonal Antibodies on Strong Cation Exchangers. Biotechnol J. 2017 Dec;12(12).
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.
Magnetic Beads Make Things Simple