- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
MKE101
BcMag™ Quick Endotoxin Removal Kit
Kit components:
2 ml: Quick Endotoxin Removal Magnetic Beads
10 ml: 10x Regeneration Buffer
2ml kit
Specification
Composition
Magnetic microsphere immobilized with Polymyxin B
Magnetization
~60 EMU/g
Type of Magnetization
Superparamagnetic
Effective Density
2.5 g/ml
Concentration
Binding Capacity
≥ 9,995 EU (endotoxin units) / ml
Storage
Ship at room temperature, Store at 4°C upon receipt
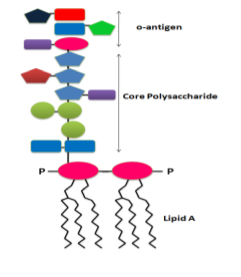
Endotoxins, also known as lipopolysaccharides, are a puzzling type of pyrogen. Their molecular structure is made up of hydrophobic fatty acid groups and hydrophilic polysaccharides, giving rise to a distinct inner core and outer region. These toxins are primarily found in the outer membranes of gram-negative bacteria such as Salmonella, E. coli, Shigella, Pseudomonas, Neisseria, Haemophilus influenzae, Bordetella pertussis, and Vibrio cholera.
When bacteria are disrupted, endotoxins are released into the environment, causing harm to the biological system. Unfortunately, endotoxins are frequently found in commercial biological products, which has a negative impact on the intended research. In the biotechnology industry, Gram-negative bacteria are commonly used to produce recombinant products such as proteins, plasmid DNAs, and vaccines, which can be contaminated with endotoxins at any stage of the process.
Endotoxins must be removed from these products because their presence can result in several pathophysiological effects such as fever, shaking chills, septic shock, toxic pneumonitis, and lethality from respiratory symptoms. The BcMag™ Quick Endotoxin Removal Kit is a novel solution that efficiently removes endotoxins via magnetic microspheres covalently immobilized with a high density of polymyxin B. Polymyxin B, a peptide antibiotic, has a high affinity for the lipid A moiety of most endotoxins.
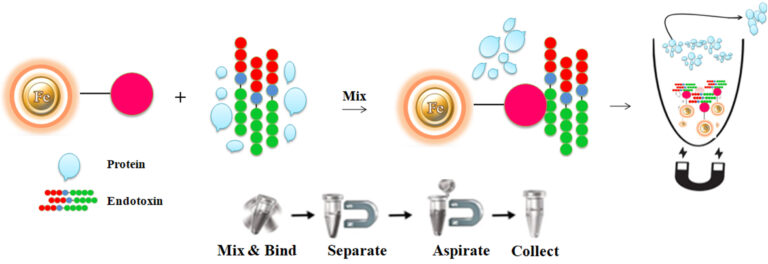
The purification with magnetic microparticles is straightforward.
1.
Mix the microparticles with the sample and incubate them with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the endotoxins to bind to the immobilized polymyxin B.
2.
After incubation, the beads are collected and separated from the sample using a magnet rack. Transfer the endotoxin-free supernatant to a fresh tube.
Materials Required
●
Regeneration Buffer: 1% Sodium deoxycholate
●
●
Magnetic rack (for manual operation)
Based on sample volume, the user can choose one of the following magnetic Racks:
– BcMag magnetic rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag magnetic rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag magnetic rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag magnetic rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ magnetic rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
For larger scale purification, Ceramic magnets Block for large scale purification ( 6 in x 4 in x 1 in block ferrite magnet, Applied Magnets, Cat. No. CERAMIC-B8).
A. Procedure
Note:
●
Equilibrate all reagents and samples to room temperature because temperature, pH, and ionic strength affect the performance of the Particles.
●
To minimize nonspecific binding, adjust all buffers to pH 7-8 and salt concentration 0.1-0.5 M NaCl (final concentration), although the Particles can bind to LPS at pH 5-9.
●
Use only endotoxin-free solutions to prevent introducing any endotoxin into the sample.
1.
Vigorously shake the bottle until the magnetic beads become homogeneous and transfer an appropriate volume of the magnetic to a new tube or flask.
Note:
2.
Place the tube on the magnetic rack for 1-3 minutes until the supernatant becomes clear. Remove the supernatant while the tube remains on the rack.
3.
4.
Repeat step (3) one more time.
5.
Resuspend the particles with ten particle-bed volumes of regeneration buffer and incubate at room temperature for 15 minutes with continuous rotation.
6.
7.
Add an appropriate amount of protein or DNA solution to the particles and incubate at room temperature for 15 minutes with continuous rotation.
8.
Place the tube on the magnetic rack for 1-3 minutes until the supernatant becomes clear. Remove the supernatant to an endotoxin-free tube while the tube remains on the rack.
B. Particles Reuse
Note:
●
For the same protein or DNA, it is possible to reuse the particles.
●
Regenerated particles may be used at least 5 times without loss of activity.
●
The Particles must be regenerated before each use, including first-time use.
1.
Regenerate particles by washing the particles with five particle-bed volumes of regeneration buffer three times to remove any bound endotoxin as described in step A(2).
2.
Wash the particles with five particle-bed volumes of endotoxin-free dH2O three times as described in step A (2).
3.
Store the particles in 20-25% ethanol at 2-8°C.
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.
Magnetic Beads Make Things Simple