- +1 858 909 0079
- +1 858 909 0057
- [email protected]
- +1 858 909 0079
- [email protected]

Products
Cat. No.
Product Name
Unit Size
Order
Specification
Composition
Magnetic beads grafted with cleavable tosyl group on the surface
Number of Beads
~ 1.68 x 109 beads/mg (1μm beads)
~1.47 x 108 beads/mg (2.5μm beads)
Stability
Short Term (<1 hour): pH 4-11; Long-Term: pH 4-10
Temperature: 4°C -140°C; Most organic solvents
Magnetization
~40-45 EMU/g
Formulation
Lyophilized Powder
Functional Group Density
1μm Magnetic Beads
~245 μmole / g of Beads
2.5μm Magnetic Beads
~230 μmole / g of Beads
Storage
Ship at room temperature. Store at -20°C, free of moisture upon receipt
BcMagTM Cleavable Tosyl-Activated Magnetic Beads are uniform magnetic beads grafted with tosyl functional groups on the surface.). The tosyl-activated magnetic beads can efficiently conjugate ligands containing primary amine in either aqueous or organic solvents (30% DMF) without introducing any charge. Since the active tosyl group is linked with the beads through a built-in cleavable disulfide linker (Fig.1), after affinity purification, reducing agents such as DTT or β-mercaptoethanol can cleave and separate the target molecule-ligand complex from the beads. The cleavable tosyl magnetic beads are ideal matrices for conjugating large-size proteins or small peptides.
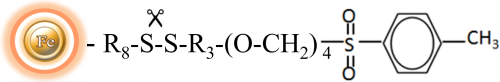
The Tosyl-Activated magnetic resins are an ideal choice for covalently attaching antibodies, peptides, complete proteins, and functional enzymes to the surface. The immobilized beads are widely used in the Immunoprecipitation of proteins and protein complexes due to their low background and covalent binding of antibodies to the bead surface.
The Tosyl Activated magnetic resins coupling reaction is carried out at 37°C, and pH ranges from neutral to high. We advocate coupling at pH 8.5-9.5, but coupling with pH labile ligands can be done in an alternate buffer at pH 7.4.
The unique dry form eliminates the need for acetone solvent storage or removal and disposal. Furthermore, because the dry resin concentrates the sample as it swells, lowering the volume of the starting material and resulting in highly effective ligand immobilization, it is perfect for coupling reactions with dilute materials.
The Beads perfectly as affinity resin for affinity purification to refine molecules, cells, and parts of cells into purified fractions. After conjugation with ligands, add the beads to a sample containing the target molecules, then mix, incubate, wash and elute the target molecules.
●
Pre-activated and ready-to-use
●
Cleavable built-in disulfide bond allowing the ligand-target molecule complex separated from the beads
●
Easy to use
●
No charge remains on the surface after coupling
●
Stable covalent bond with minimal ligand leakage
●
Produces reusable immunoaffinity matrix
●
Low nonspecific binding
●
Immobilize 1-10 mg protein or 0.1-1 mg peptide/ml beads
●
Applications: Immunoprecipitation; Purification for Antibodies, Proteins/Peptides, DNA/RNA
Note:
●
This protocol can be scaled up as needed. We strongly recommended titration to optimize the number of beads used for each application.
●
Avoid reducing agents, tris, or other buffers containing primary amines or other nucleophiles because these will break the disulfide linker or compete with the intended coupling reaction. But the wash or storage buffers can have amino or carboxyl groups.
Materials Required
1.
Magnetic Rack (for manual operation)
Based on sample volume, the user can choose one of the following Magnetic Racks:
– BcMag™ Magnetic Rack-2 for holding two individual 1.5 ml centrifuge tubes (Cat. No. MS-01);
– BcMag™ Magnetic Rack-6 for holding six individual 1.5 ml centrifuge tubes (Cat. No. MS-02);
– BcMag™ Magnetic Rack-24 for holding twenty-four individual 1.5-2.0 ml centrifuge tubes (Cat. No. MS-03);
– BcMag™ Magnetic Rack-50 for holding one 50 ml centrifuge tube, one 15 ml centrifuge tube, and four individual 1.5 ml centrifuge tubes (Cat. No. MS-04);
– BcMag™ Magnetic Rack-96 for holding a 96 ELISA plate or PCR plate (Cat. No. MS-05).
2.
Coupling Buffer: 0.1 M sodium phosphate, pH 7.4
Note:
●
The coupling buffers should be minimal ionic strengths and should not contain any amino (e.g., Tris or glycine). But the wash or storage buffers can have amino or carboxyl groups.
●
Water-insoluble ligands can be conjugated in 30% organic solvent (30% DMF) with a coupling buffer.
3.
Blocking Buffer: PBS pH 7.4 with 0.5% (w/v) BSA
4.
Washing buffer: PBS pH 7.4 with 0.1% (w/v) BSA.
A.
Magnetic Beads Preparation
1.
Prepare 3% magnetic beads with 100% isopropanol (30 mg/ml). Note: Store the unused beads in acetone solution at 4°C. It is stable for over a year.
2.
Transfer 100 μl (3mg) magnetic beads to a centrifuge tube.
3.
Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack. Remove the tube from the rack and resuspend the beads with 1 ml coupling buffer by vortex for 30 seconds.
4.
Repeat step 3 two times.
5.
Remove the supernatant, and the washed beads are ready for coupling.
Note: Once rehydrated, use the bead as soon as possible due to the stability of the functional group.
B.
Protein Coupling
1.
Prepare 100 μl of protein solution (0.5-1mg/ml) or peptide solution (200 μmoles/ml) with coupling buffer.
Note: Coupling efficiencies vary from ligand to ligand. The user should empirically optimize the concentration of the ligand.
2.
Add the protein or peptide solution to the washed beads and mix well by vortex or pipette.
3.
Incubate the reaction 24-48 hours at 20-25°C or 48-72 hours at 4°C with continuous rotation.
4.
Wash beads three times with 1 ml washing buffer.
5.
Add 1ml blocking buffer to the beads and incubate at room for 1 hour or at 4 °C overnight.
6.
Wash beads 4-6 times with 1 ml PBS buffer.
7.
Resuspend the beads in PBS buffer with 0.01% azide (w/v) to desired concentration and store at 4°C until use. Do not freeze.
C.
General Affinity Purification Protocol
Note:
●
This protocol is a general affinity purification procedure. Designing a universal protocol for all protein purification is impossible because no two proteins are precisely alike. The user should determine the optimal working conditions for purifying the individual target protein to obtain the best results.
●
Avoid reducing agents in binding and washing buffers.
●
We strongly recommended titration to optimize the number of beads used for each application based on the amount of the target protein in the crude sample. Too many magnetic beads used will cause higher backgrounds, while too few beads used will cause lower yields. Each mg of magnetic beads typically binds to 10-20 μg of the target protein.
1.
Transfer the optimal amount of the beads to a centrifuge tube. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
2.
Remove the tube and wash the beads with 5-bed volumes of PBS buffer by vortex for 30 seconds. Leave the tube at room temperature for 1-3 minutes. Place the tube on the magnetic rack for 1-3 minutes. Remove the supernatant while the tube remains on the rack.
3.
Repeat step 2 two times.
4.
Add washed beads to the crude sample containing the target protein and incubate at room or desired temperature for 1-2 hours (Lower temperatures require longer incubation time).
Note: Strongly recommended to perform a titration to optimize incubation time. More prolonged incubation may cause higher background.
5.
Note: Adding a higher concentration of salts, nonionic detergent, and reducing agents may reduce the nonspecific background. For example, adding NaCl (up to 1-1.5 M), and 0.1-0.5% nonionic detergents such as Triton X100 or Tween20 to the washing buffer.
6.
Elute the target protein by appropriate methods such as low pH (2-4), high pH (10-12), high salt, high temperature, affinity elution, or boiling in SDS-PAGE sample buffer, or reducing agents.
7.
Cleave the Disulfide Bond
Note: Due to conformational variation from ligands to ligands, the user should determine the optimal working conditions such as reducing agent, pH, and temperature for cleaving the disulfide bond of individual ligands. The following is an example of cleaving conjugated GFP from the beads.
●
Incubate the magnetic beads (30mg/ml) in either 140 mM β-mercaptoethanol or 5mM DTT (Dithiothreitol)
a. 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 140 mM β-mercaptoethanol for 2 hours to overnight at room temperature or 98°C for 5 minutes.
b. 100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 5mM DTT for 2 hours to overnight at room temperature or 98°C for 5 minutes.
Get the Latest News and Updates by Email
6393 Nancy Ridge Dr. Suite A
San Diego, CA 92121 USA
Fax: +1-858-909-0057
Get the Latest News and Updates by Email
© 2023 Bioclone Inc. All Rights Reserved.